Use of Complementary and Alternative Medicine by Oncology Patients in the City of Bucaramanga, Colombia
Uso de Medicina Complementaria y Alternativa en Pacientes Oncológicos en la Ciudad de Bucaramanga, Colombia
Nicolás Martínez-Ramos , Norma Cecilia Serrano Díaz, Claudia Janeth Uribe-Pérez, Doris Cristina Quintero-Lesmes, Carolina Mariño-Manrique, Raúl Murillo
Abstract
Introduction. Complementary and alternative medicine (CAM) is frequently used by oncology patients worldwide, often alongside conventional treatments. Understanding the frequency of use and motivations for CAM use is essential to improve patient-centered cancer care and guidance.
Objective. To determine the frequency of use of complementary and alternative medicine (CAM) in oncology patients in the city of Bucaramanga, Colombia.
Method. Descriptive study; patients were surveyed at a health center in Bucaramanga, Colombia. Adult patients with any type of cancer, at all clinical stages, and who were undergoing active treatment were included.
Results. A total of 528 patients were included. Seventy-one percent were women, with an average age of 56.6 years. The most common cancer diagnoses were breast, colon, and stomach neoplasms. The CAM’s frequency of use was 67%, the majority of whom were women and patients with an educational level close to primary school. The primary reason for use was for palliative purposes, but a significant number used it for a curative purpose. The most commonly used products by patients were special foods of natural origin, including soursop and soursop leaves. As the main source of information about CAM, 86% reported obtaining this information from people other than the healthcare team.
Conclusion. A high proportion of oncology patients undergoing active treatment use CAM. It is necessary to incorporate this information into patient care and to develop services that guide such practices.
Keywords
Cancer; Colombia; complementary therapies; integrative oncology; surveys and questionnaires.
Resumen
Introducción. La medicina complementaria y alternativa (MCA) es frecuentemente utilizada por pacientes oncológicos en todo el mundo, a menudo junto con tratamientos convencionales. Comprender la frecuencia de uso y las motivaciones para el uso de MCA es esencial para mejorar la atención oncológica centrada en el paciente y la orientación adecuada.
Objetivo. Determinar la frecuencia de uso de medicina complementaria y alternativa (MCA) en pacientes oncológicos en la ciudad de Bucaramanga, Colombia.
Método. Estudio descriptivo; los pacientes fueron encuestados en un centro de salud en Bucaramanga, Colombia. Se incluyeron pacientes adultos con cualquier tipo de cáncer, en todos los estadios clínicos, que estuvieran recibiendo tratamiento activo.
Resultados. Se incluyeron un total de 528 pacientes. El setenta y uno por ciento eran mujeres, con una edad promedio de 56,6 años. Los diagnósticos de cáncer más comunes fueron neoplasias de mama, colon y estómago. La frecuencia de uso de MCA fue del 67%, la mayoría de los cuales eran mujeres y pacientes con un nivel educativo cercano a la educación primaria. La principal razón de uso fue con fines paliativos, aunque un número significativo la utilizó con un propósito curativo. Los productos más comúnmente usados fueron alimentos especiales de origen natural, incluyendo la guanábana y sus hojas. Como principal fuente de información sobre MCA, el 86% reportó obtener esta información de personas ajenas al equipo de salud.
Conclusión. Una alta proporción de pacientes oncológicos que reciben tratamiento activo usan MCA. Es necesario incorporar esta información en la atención al paciente y desarrollar servicios que orienten tales prácticas.
Palabras clave
Cáncer; Colombia; terapias complementarias; oncología integrativa; encuestas y cuestionarios.
Introduction
Cancer is a growing public health problem in Colombia, considered the second leading cause of death after cardiovascular diseases, with more than 100,000 new cases per year [1]. The threatening nature of this disease has been a determining factor for the use of complementary and alternative medicine (CAM), as evidenced by the high frequency of use reported in oncology patients compared to other patients with chronic conditions [2]. CAM is defined as a group of medical and healthcare systems that involve practices and products that are not considered part of conventional medicine, which includes mind-body therapies, biological based practices, energetic CAM, whole medical systems, and body manipulation [3].
Worldwide, a frequency of use ranging from 9.8% to 76% has been found in oncology patients [4]. In Colombia, the National Cancer Institute reports that 73.5% of its adult patients and 81.9% of the caregivers of pediatric patients use CAM [5,6]. The main reasons for using CAM are related to palliative purposes, their "natural" character, and their positive effects on quality of life [7-9]. However, many users of CAM obtain information about these practices from people other than medical professionals [10], avoiding communicating its use to their treating oncology physicians [11], often unaware of the potential side effects that may arise from their concurrent use with conventional treatment [7,8,12,13]. In that manner, it has been found that certain foods can interact with medications used in conventional treatment, for example, turmeric improves antitumor activity [14], and the Guinea Henweed has shown to reduce tumor burden, metastasis, and to improve the peripheral immune response in murine myeloid leukemia models [15].
Bucaramanga is one of the cities in the country with a Cancer Population Registry [13] that reports a growing trend in the incidence of various cancer diagnoses [16,17], in which were reported 3,801 new cases of cancer (excluding non-melanoma skin cancer) by 2016, with an incidence rate similar to that of the rest of the country [18], in addition to the increasing number of cases due to the aging population, and some of the health barriers presented by gastric oncology patients in the city [19] make it worth to study the frequency of use of alternative and complementary medicines among oncology patients in the city, information that is crucial for appropriate oncology care planning.
Therefore -and given that patients often do not discuss this issue with their treating physicians [11,20-22], and that the objective of this study was to characterize the frequency, reasons for use, and specific practices of oncology patients regarding CAM in the city of Bucaramanga, Colombia-, we hope to expand with the results the current knowledge on the use of CAM in Colombia and in the department of Santander, which involves as well the reasons for use and the main products used by the patients, under the concept of integrative medicine. Consequently, CAM therapies are integrated into the main course of treatment [3].
Method
A descriptive cross-sectional study was conducted and approved by the ethics committees of Hospital San Ignacio/Pontificia Universidad Javeriana in Bogotá and Fundación Cardiovascular de Colombia in Bucaramanga. All participants gave their consent to participate.
The methodology has been described previously [23]. A survey was designed based on the classification of CAM proposed by the National Center for Complementary and Integrative Health [24] and the results of focus groups that explored the types of practices and reasons for the use of CAM among oncology patients [25], that could represent the total CAM domains. The survey included sociodemographic data, clinical characteristics, frequency of CAM use according to standard categories, reasons for use, and specific practices.
A sample size of 525 subjects was defined based on an expected CAM’s frequency of use of 70% [26], A 95% confidence interval, a 5% precision, and a design effect of 2 were used. Sampling was carried out using random days, with the recruitment day considered a cluster and an expected intraclass correlation of 3.5%, which led to the need for at least 35 clusters with blocks of 15 observations each. Various institutions in the city were invited to participate, but only Hospital Internacional de Colombia of Fundación Cardiovascular de Colombia participated.
Patients over 18 years old with a histopathological diagnosis of cancer, who were undergoing active treatment with chemotherapy (systemic treatment) or had received radiotherapy or surgery in the past 4 months, were included. Patients at any clinical stage and with any type of cancer were included. Patients who could not answer the questionnaire due to their physical or mental condition were excluded, and participation by family members or caregivers on behalf of the patients was not accepted. The information was recorded on the RedCap platform [27] by pollsters previously trained. A pilot was conducted by 10 patients, in which the compressibility of the survey was tested. Also, after collecting the data, 10% of the patients was called randomly in order to verify the quality of data.
Absolute frequencies, relative frequencies, and measures of central tendency were used for numerical variables and data analysis. The frequency of use was reported as a percentage, and clinical and sociodemographic characteristics between CAM users and non-users were compared using the chi-square test and the Kruskal-Wallis test, according to the data distribution and the nature of the variables. The significance level was set at a p-value of <0.05. Specific practices reported in open-ended questions were coded and grouped according to the basic description of the products (biological or processed) or practices (mind-body and others). Once coded and grouped, absolute and relative frequencies were measured and compared based on their palliative or curative purposes. The analysis was conducted using the Python® statistical package.
Institutional Review Board Statement
The study was conducted by the Declaration of Helsinki, and approved by the institutional ethics review board Hospital San Ignacio/Pontificia Universidad Javeriana, according to Act No. FM-CIE-004-18 of 2018.
Informed Consent Statement
Written informed consent was obtained directly from all participants.
Results
A total of 528 patients with an active diagnosis participated, with a higher participation of women, patients with subsidized health insurance, and patients with a lower socioeconomic status. The most commonly reported type of treatment was chemotherapy (Table 1). Additionally, the most frequent type of cancer was breast cancer, followed by colon cancer and stomach cancer (Supplementary Table 1).
Table 1. Description of Sociodemographic and Clinical Variables versus CAM Use.
| Variables | Total | CAM Users | CAM No Users | p-value | |
|---|---|---|---|---|---|
| N | 528 | 352 | 176 | ||
| Age | Mean | 56.6 | 56 | 58 | 0.131 |
| SD | 14.2 | 13.8 | 14.9 | ||
| n (%) | n (%) | n (%) | |||
| Sex | Women | 375 (71.0) | 255 (72.4) | 120 (68.2) | 0.360 |
| Men | 153 (29.0) | 97 (27.6) | 56 (31.8) | ||
| Religion | Catholic | 385 (73.1) | 259 (73.6) | 126 (72.0) | 0.335 |
| Christian | 108 (20.5) | 72 (20.5) | 37 (21.0) | ||
| Other | 11 (2.1) | 9 (2.6) | 2 (1.1) | ||
| No Religion | 23 (4.4) | 12 (3.4) | 11 (6.2) | ||
| Marital Status | Single | 152 (28.8) | 100 (28.4) | 52 (28.4) | 0.888 |
| Married | 187 (35.5) | 122 (34.7) | 65 (36.9) | ||
| Free union | 101 (19.1) | 69 (19.6) | 32 (19.6) | ||
| Divorced | 41 (7.8) | 30 (8.5) | 11 (8.5) | ||
| Widowed | 47 (8.9) | 31 (8.8) | 16 (8.8) | ||
| Socioeconomic Status | Low | 381(72.2) | 248(70.5) | 133(72.2) | 0.155 |
| Medium | 142(26.9) | 102(29.9) | 40(27.7) | ||
| High | 5(0.9) | 2(0.6) | 3(1.7) | ||
| Occupation | Unemployed | 54 (10.2) | 37 (10.5) | 17 (9.7) | 0.835 |
| Employed | 43 (8.1) | 26 (7.4) | 17 (9.7) | ||
| Student | 7 (1.3) | 4 (1.1) | 3 (1.7) | ||
| Homemaker | 314 (59.5) | 207 (58.8) | 107 (60.8) | ||
| Self-Employed | 78 (14.8) | 55 (15.6) | 23 (13.1) | ||
| Retired | 32 (6.1) | 23 (6.5) | 9 (5.1) | ||
| Educational Level | None - Primary Education | 237(44.9) | 139(39.5) | 98(55.7) | 0.004 |
| High School | 201(38.1) | 144(40.9) | 57(32.4) | ||
| Technical/Technological | 54(10.2) | 40(11.4) | 14(8.0) | ||
| University - Postgraduate | 36(6.8) | 7(4.0) | 29(8.2) | ||
| Health Insurance | Contributory | 170 (32.2) | 113 (32.1) | 57 (32.4) | 0.950 |
| Subsidized | 345 (65.3) | 230 (65.3) | 115 (65.3) | ||
| Complementary Plan | 8 (1.5) | 6 (1.7) | 2 (1.1) | ||
| Other | 5 (0.9) | 3 (0.9) | 2 (1.1) | ||
| Cancer Stage | Localized | 279(52.8) | 191(54.3) | 88(50.0) | 0.093 |
| Lymph Nodes Involved | 103(19.5) | 68(19.3) | 35(19.9) | ||
| Metastasis | 134(25.4) | 89(25.3) | 45(25.6) | ||
| Most Common Cancer Diagnosis | Breast | 166 | 124(35.2) | 42(12.0 ) | >0.05 |
| Colon | 49 | 36(10.2) | 13(3.7) | ||
| Stomach | 36 | 19(5.4) | 17(4.8) | ||
| Ovary | 31 | 24(6.8) | 7(2.0) | ||
| Cervix | 26 | 15(4.3) | 11(3.1) | ||
| Treatment-Related Side Effects | Yes | 300(72.0) | 260(73.9) | 120(68.2) | 0.205 |
| No | 148(28.0) | 92(26.1) | 56(31.8) | ||
| Treatments Received | Chemotherapy | 438(83.5) | 296 (84.1) | 142 (80.7) | 0.038 |
| Radiotherapy | 163(30.9) | 112(31.8) | 51 (29.0) | 0.890 | |
| Surgery | 221(41.9) | 149 (42.61) | 72 (41.0) | 0.023 | |
| Palliative Care | 1(0.2) | - | 1(0.6) | 0.968 | |
Note. SD (Standard Deviation). Note: For the age variable, the Kruskal-Wallis test was used due to the nature of the variable and the non-normal distribution of the data. For other variables, the chi-square test was used. Bold text indicates statistically significant values.
A total of 67% of the surveyed patients reported using CAM. CAM users had a higher educational level and more frequently received systemic treatment and surgery (Table 1). The use of biologically based medicines, especially natural origin foods, was found to be more common, whereas the use of medicine based on alternative medical systems was less frequent. The primary reason for use was related to palliative goals; patients reported that they mostly obtain information about CAM from sources other than their healthcare team, with few patients discussing this topic with their doctors. A high percentage of patients reported having a favorable experience with CAM use, perceiving it as low-cost (Table 2).
Table 2. Practices of Alternative and Complementary Medicine (CAM) Use.
| Items Describing CAM Use | % | ||
|---|---|---|---|
| CAM Types | Biological Basis | Special Foods of Natural Origin | 64% |
| Special Diet | 14% | ||
| Herbal Products | 55% | ||
| Animal-Based Products | 32% | ||
| Products Intended to Supplement Nutrition | Vitamins and Supplements | 48% | |
| Alternative Medical Systems | Homeopathy | 11% | |
| Traditional Practices | 1.7% | ||
| Acupuncture | 0.3% | ||
| Other Therapies | Massage | 3% | |
| Energy Therapy | 3% | ||
| Mind-Body Therapy | 2% | ||
| Reasons for Use | Palliation | Counteracting Adverse Effects of Treatment or Disease | 52% |
| Emotional Support | 8% | ||
| Disease Control | Cure the Disease | 20% | |
| Prevent Recurrence | 16% | ||
| Toxicity | Its Natural Properties Compared to Conventional Medicine | 5% | |
| Others | Exploring All Available Options to Treat the Disease | 3% | |
| Other Reason | 28% | ||
| Initial Source of Information | Medical Recommendation (Conventional or Alternative) | 7% | |
| Another Person Outside the Medical Team (Family, Friends, Patients, Pharmacists, Marketplaces) | 86% | ||
| Media | 4% | ||
| Other | 3% | ||
| Effects of CAM Use | Favorable | 85% | |
| Indifferent | 13% | ||
| Unfavorable | 1% | ||
| Perception of CAM Costs | High Cost | 33% | |
| Medium Cost | 26% | ||
| Low Cost | 41% | ||
| Discussion with Treating Physician about CAM | Yes | 35% | |
| No | 65% | ||
| Reasons for Not Discussing It | No Need | 21% | |
| The Doctor Did Not Ask | 64% | ||
| Possible Disapproval from the Doctor | 13% | ||
| Other | 2% | ||
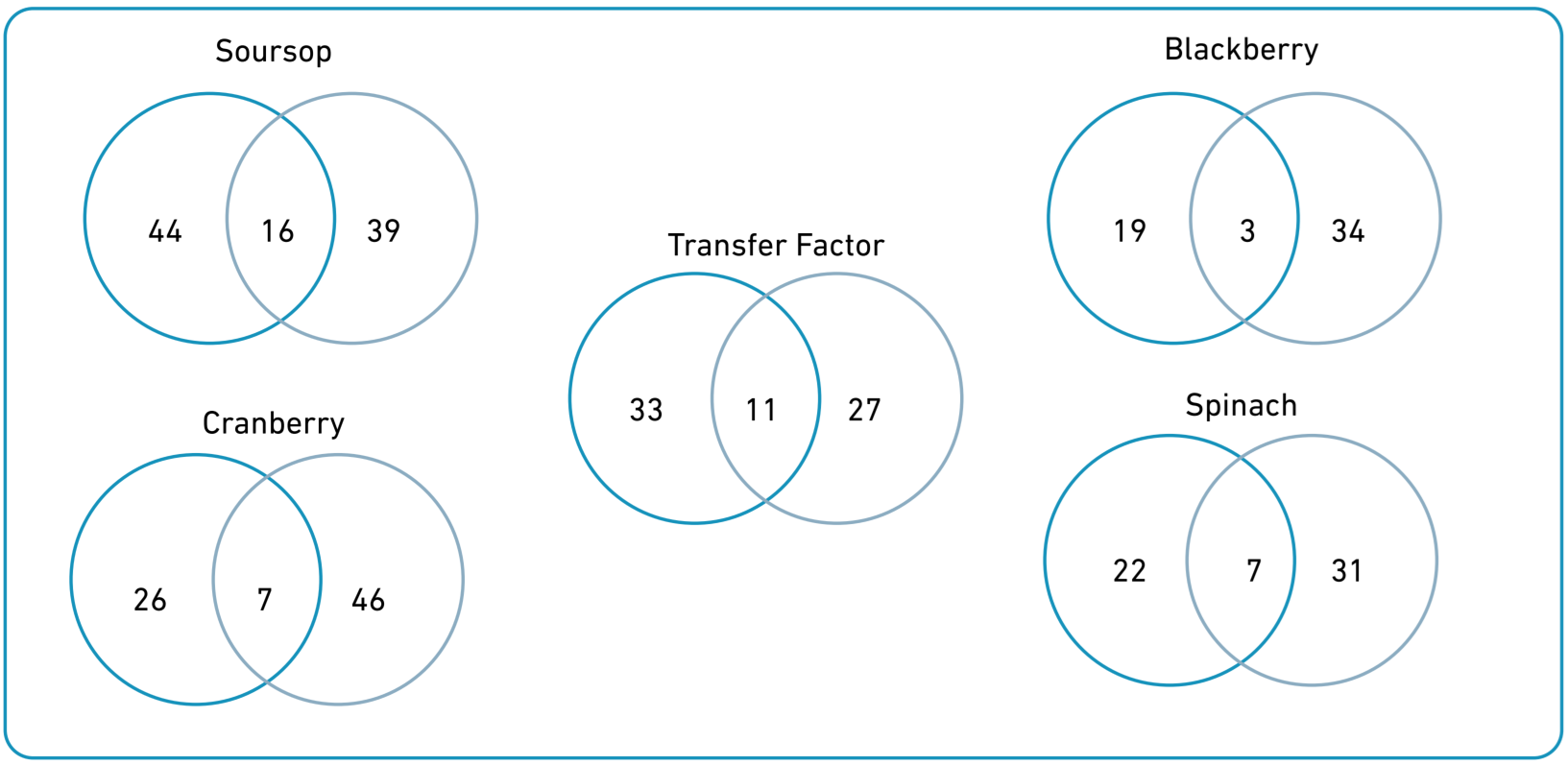
Soursop was the most reported product (36%), followed by cranberry (31%), transfer factors (27), blackberry (26%), and spinach (24%). Soursop and transfer factors had a greater frequency for curative than palliative purposes, whereas the opposite was observed for blackberry, cranberry, and spinach (Figure 1). The reasons for use of the 15 most frequent products are described in Supplementary Table 2.
Figure 1. Venn Diagrams; gray circles = palliative purposes, turquoise circles = curative purposes.
Discussion
Our study found a high CAM’s frequency of use among oncology patients in Bucaramanga (67%), a value consistent with reports from Chile [28], but lower than findings in other Latin American contexts, such as Argentina and Bogotá, Colombia [6,29]. Additionally, the frequency of use reported in European countries is generally lower [9,30-32]. This variation highlights the influence of sociocultural and healthcare system factors on adopting CAM, as has been described in comparative studies [33]. In Latin America, the accessibility and affordability of CAM, coupled with traditional beliefs and a fragmented healthcare system, may contribute to its widespread use, whereas in European countries, stricter regulatory frameworks and integrative medicine models shape different utilization patterns [33].
The data were obtained from a single treatment center, similar to studies conducted in Bogotá and Chile. Therefore, the results should be interpreted cautiously in terms of their representativeness of the broader oncology patient population in the city. Beyond this, the differences in usage frequency could originate from sociodemographic and cultural patterns that influence perceptions of CAM [24,34]. The majority of CAM users were women, which aligns with previous studies showing that women have a significantly higher frequency of use [31-36], likely related to a more favorable stance towards CAM and an understanding of their health condition from various dimensions [37]. This gender disparity is a well-documented trend in CAM research and may be explained by a greater willingness among women to explore non-conventional therapeutic options, as well as a higher perceived need for holistic approaches to health [38]. Moreover, gendered health behaviors and decision-making patterns should be considered in the development of CAM-related interventions and policies.
Most CAM users in this study were patients with a low educational level and socioeconomic status, reflecting the overall behavior of the sample. However, CAM users had a higher educational level than non-users, with a statistically significant association, which was not observed for socioeconomic status (Table 1). This result is partially similar to what has been previously reported, as CAM users tend to have higher levels of education but also higher income levels [7,31,36,39,40]. Our finding is possibly related to the high frequency of use of expensive products such as transfer factors, which are marketed for widespread use but might be more accepted and affordable for individuals with higher educational levels. This paradox-where higher education is linked to greater CAM use despite economic constraints-suggests that education fosters health-seeking behaviors that include CAM, while financial limitations shape the type of CAM therapies accessed [39].
The frequent use of biologically based products could be associated with the perception of lower risk, as they are considered natural [7-9,41]. However, it has been observed that garlic, ginkgo, St. John's wort, and kava can negatively interact with anticancer medications [42]. On the other hand, frequently used products in this category, such as soursop, blackberry, and cranberry, have shown divergent results in experimental models, ranging from neurotoxicity in soursop experiments to antioxidant effects in cranberry studies [43-45]. For other commonly used products like transfer factors, we did not find information regarding their pharmacokinetic potential or interactions with anticancer treatments. This underscores a critical gap in the evidence base for many widely used CAM therapies, reinforcing the need for rigorous pharmacological studies and clinical trials to elucidate both efficacy and safety profiles [33]. Additionally, this highlights the necessity of healthcare provider training in CAM-related counseling to mitigate risks associated with its unsupervised use.
The most frequently reported reasons for use were palliative purposes, which aligns with findings from other studies [32,36]. Patients generally do not use CAM as a replacement for their conventional treatment; rather, they use it to gain better control over their health condition and to approach their illness from a psychological perspective [9,46]. In this same vein, the primary source of information for patients was their family and friends, which aligns with the conceptualization of CAM as stemming from popular knowledge [24,30,32,34,44]. However, in higher-income countries, it is assumed that the use of CAM should originate from a medical recommendation [32]. This disparity in information sources suggests a need for educational interventions aimed at improving patient awareness and encouraging dialogue with healthcare providers about CAM use. In countries like Colombia, where physician knowledge of CAM is often limited, capacity-building programs within oncology services could facilitate evidence-based guidance on CAM therapies. The integration of CAM into conventional healthcare settings requires not only patient education, but also structured training programs for medical professionals to ensure safe and effective recommendations [47].
Our study has several limitations. Conducting it at a single healthcare institution limits its external validity, even though the center where the study was conducted serves a significant proportion of cancer cases in the city and the region. The results should, therefore, be understood in relation to the characteristics of the study population, which includes a higher proportion of patients with subsidized health coverage, correlating with lower educational levels and fewer resources. However, some of our findings, such as the educational level, showed independence from the effect of these variables. On the other hand, the results reported by patients may be subject to memory bias or social desirability. Nevertheless, the presence of an interviewer has shown higher reporting of CAM use compared to when patients self-administer the survey [48]. Future studies should consider longitudinal designs to assess changes in CAM use over time, as well as qualitative approaches to understand the motivations, experiences, and decision-making processes of oncology patients regarding CAM. Integrating CAM research into national cancer control strategies could also support the development of policies that address both safety concerns and patient preferences.
The results of our study highlight the need for increased research into CAM, particularly the development of clinical studies that can support the therapeutic potential or drug interactions of products frequently used by patients in this region. Additionally, the high frequency of CAM use underscores the need to strengthen integrative medicine services, making our study's contribution relevant to this purpose. Given the widespread use of CAM among oncology patients, integrating CAM discussions into routine oncology consultations could enhance patient-provider communication and ensure safer, evidence-based practices. Moreover, public health strategies should focus on regulating and standardizing CAM products and services to align with national healthcare objectives while respecting cultural health beliefs.
Conclusions
In conclusion, this study demonstrates a widespread use of complementary and alternative medicine (CAM) among oncology patients in Bucaramanga, reflecting a trend toward complementing conventional treatment. Integrating integrative medicine services is essential to optimize oncology care.
References
1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Globocan 2022 [Internet]. Lyon: International Agency for Research on Cancer; 2024 [cited 2024 Nov 20]. 1 p. Available from:
2. Tangkiatkumjai M, Boardman HF, Walker DM. Potential factors that influence usage of complementary and alternative medicine worldwide: a systematic review. BMC Complement Med Ther [Internet]. 2020;20(1):363. doi: https://doi.org/10.1186/s12906-020-03157-2
3. National Center for Complementary and Integrative Health (NCCIH) [Internet]. Bethesda: National Institutes of Health (NIH); c2025. Statistics on Complementary and Integrative Health Approaches; 2025 Mar 3 [cited 2025 Feb 3]; [about 2 screens]. Available from:
4. Harris PE, Cooper KL, Relton C, Thomas KJ. Prevalence of complementary and alternative medicine (CAM) use by the general population: a systematic review and update. Int J Clin Pract [Internet]. 2012;66(10):924-39. doi: https://doi.org/10.1111/j.1742-1241.2012.02945.x
5. Sánchez R, Ibáñez CPB, Suárez A. Utilización de terapias complementarias y alternativas en niños con cáncer. Rev salud pública [Internet]. 2015;17(5):699-712. doi: https://doi.org/10.15446/rsap.v17n5.38695
6. Sánchez R, Venegas M. Aproximaciones complementarias y alternativas al cuidado de la salud en el Instituto Nacional de Cancerología: estudio de prevalencia. Rev Colomb Cancerol [Internet]. 2010;14(3):135-43. doi: https://doi.org/10.1016/S0123-9015(10)70094-9
7. Abuelgasim KA, Alsharhan Y, Alenzi T, Alhazzani A, Ali YZ, Jazieh AR. The use of complementary and alternative medicine by patients with cancer: a cross-sectional survey in Saudi Arabia. BMC Complement Altern Med [Internet]. 2018;18(1):88. doi: https://doi.org/10.1186/s12906-018-2150-8
8. Guerra-Martín MD, Tejedor-Bueno MS, Correa-Casado M. Effectiveness of Complementary Therapies in Cancer Patients: A Systematic Review. Int J Environ Res Public Health [Internet]. 2021;18(3):1-10. doi: https://doi.org/10.3390/ijerph18031017
9. Kristoffersen AE, Nilsen JV, Stub T, Nordberg JH, Wider B, Mora DC, et al. Use of Complementary and Alternative Medicine in the context of cancer; prevalence, reasons for use, disclosure, information received, risks and benefits reported by people with cancer in Norway. BMC Complement Med Ther [Internet]. 2022;22(1):202. doi: https://doi.org/10.1186/s12906-022-03606-0
10. Adams M, Jewell A. The use of complementary and alternative medicine by cancer patients. Int Semin Surg Oncol [Internet]. 2007;4:10. doi: https://doi.org/10.1186/1477-7800-4-10
11. García-Padilla P, García-Padilla D, Ramírez-Castro MF, Pulido-Rincón P, Murillo R. Patient-doctor interactions around alternative and complementary medicine in the context of oncology care in a Latin American country. Complement Ther Med [Internet]. 2023;78:102986. doi: https://doi.org/10.1016/j.ctim.2023.102986
12. Mora DC, Kristoffersen AE, Overvåg G, Jong MC, Mentink M, Liu J, et al. Safety of Complementary and Alternative Medicine (CAM) treatment among children and young adults who suffer from adverse effects of conventional cancer treatment: A systematic review. Integr Cancer Ther [Internet]. 2022;21. doi: https://doi.org/10.1177/15347354221105563
13. Sweet ES, Standish LJ, Goff BA, Andersen MR. Adverse Events Associated With Complementary and Alternative Medicine Use in Ovarian Cancer Patients. Integr Cancer Ther [Internet]. 2013;12:508-16. doi: https://doi.org/10.1177/1534735413485815
14. Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a Dietary Component, Has Anticancer, Chemosensitization, and Radiosensitization Effects by Down-regulating the MDM2 Oncogene through the PI3K/mTOR/ETS2 Pathway. Cancer Res [Internet]. 2007;67(5):1988-96. doi: https://doi.org/10.1158/0008-5472.CAN-06-3066
15. Murillo N, Lasso P, Urueña C, Pardo-Rodriguez D, Ballesteros-Ramírez R, Betancourt G, et al. Petiveria alliacea Reduces Tumor Burden and Metastasis and Regulates the Peripheral Immune Response in a Murine Myeloid Leukemia Model. Int J Mol Sci [Internet]. 2023;24(16):12972. doi: https://doi.org/10.3390/ijms241612972
16. Bravo LE, Muñoz N. Epidemiology of cancer in Colombia. Colomb Med[Internet]. 2018;49(1):9-12. doi: https://doi.org/10.25100/cm.v49i1.3877
17. Uribe Pérez CJ, Serrano-Gómez S, Hormiga Sánchez CM. Cancer incidence and mortality in Bucaramanga, Colombia. 2008-2012. Colomb Med [Internet]. 2018;49(1):73-80. doi: https://doi.org/10.25100/cm.v49i1.3632
18. Pardo C, Cendales R. Incidencia, mortalidad y prevalencia de cáncer en Colombia, 2012-2016. Vol 1 [Internet]. Bogotá: Instituto Nacional de Cancerología; 2022 [cited 2025 Feb 3]. 159 p. Available from:
19. Uribe Pérez CJ, Amado Niño AM, Rueda Patiño AM. Barreras para la atención en salud del cáncer gástrico, Santander, Colombia. Etapa exploratoria. Rev Colomb Gastroenterol [Internet]. 2019;34(1):17-22. doi: https://doi.org/10.22516/25007440.353
20. Källman M, Bergström S, Carlsson T, Järås J, Holgersson G, Nordberg JH, et al. Use of CAM among cancer patients. BMC Complement Med Ther [Internet]. 2023;23(1):51. doi: https://doi.org/10.1186/s12906-023-03876-2
21. Stub T, Quandt SA, Kristoffersen AE, Jong MC, Arcury TA. Communication and information needs about complementary and alternative medicine: a qualitative study of parents of children with cancer. BMC Complement Med Ther [Internet]. 2021;21(1):85. doi: https://doi.org/10.1186/s12906-021-03253-x
22. Wang SY, Lin LW, Chang YY, Huang YP. Health care professionals’ interactions with cancer patients who use complementary and alternative medicine in Taiwan. Collegian [Internet]. 2016;23(2):209-16. doi: https://doi.org/10.1016/j.colegn.2015.02.007
23. Murillo R, Pinto-Martínez N, Serrano N, Uribe C, Navarro E, Duque J, et al. Use of complementary and alternative medicine by cancer patients in Colombia. BMC Complement Med Ther [Internet]. 2023;23(1):321. doi: https://doi.org/10.1186/s12906-023-04144-z
24. National Center for Complementary and Integrative Health (NCCIH) [Internet]. Bethesda: National Institutes of Health (NIH); c2025. Salud complementaria, alternativa o integral: ¿Qué hay detrás de estos nombres? 2025 Mar 3 [cited 2025 Feb 3]; [about 12 screens]. Available from:
25. García-Padilla P, Ordóñez Reyes C, Medina P, Fernández Deaza G, Morales OL, Murillo R. Perspectivas de pacientes y profesionales en torno al uso de medicinas alternativas y complementarias para el cuidado del cáncer: un estudio exploratorio. Univ Med [Internet]. 2021;62(1):1-12. doi: https://doi.org/10.11144/javeriana.umed62-1.pppu
26. Zapata-Ossa H de J, Cubides-Munévar AM, López MC, Pinzón-Gómez EM, Filigrana-Villegas PA, Cassiani-Miranda CA. Muestreo por conglomerados en encuestas poblacionales. Rev salud pública [Internet]. 2011;13(1):141-51. Available from: https://revistas.unal.edu.co/index.php/revsaludpublica/article/view/33543
27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform [Internet]. 2009;42(2):377-81. doi: https://doi.org/10.1016/j.jbi.2008.08.010
28. Lopez G, Salas CA, Cadiz F, Barriga C, Gonzalez P, Acevedo S, et al. Complementary and Integrative Medicine Use in Individuals Seeking Conventional Medical Oncology Care in Chile: Prevalence and Patient Characteristics. J Glob Oncol [Internet]. 2019;5:1-6. doi: https://doi.org/10.1200/JGO.18.00190
29. Idoyaga Molina N, Luxardo N. Medicinas no convencionales en cáncer. Med B Aires [Internet]. 2005;65(5):390-4. Available from: https://www.scielo.org.ar/scielo.php?pid=S0025-76802005000500002&script=sci_abstract&tlng=en
30. Bahall M. Prevalence, patterns, and perceived value of complementary and alternative medicine among cancer patients: a cross-sectional, descriptive study. BMC Complement Altern Med [Internet]. 2017;17(1):345. doi: https://doi.org/10.1186/s12906-017-1853-6
31. Molassiotis A, Fernadez-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol [Internet]. 2005;16(4):655-63. doi: https://doi.org/10.1093/annonc/mdi110
32. Wode K, Henriksson R, Sharp L, Stoltenberg A, Nordberg JH. Cancer patients’ use of complementary and alternative medicine in Sweden: a cross-sectional study. BMC Complement Altern Med [Internet]. 2019;19(1):62. doi: https://doi.org/10.1186/s12906-019-2452-5
33. Romero-García PA, Ramirez-Perez S, Miguel-González JJ, Guzmán-Silahua S, Castañeda-Moreno JA, Komninou S, et al. Complementary and Alternative Medicine (CAM) Practices: A Narrative Review Elucidating the Impact on Healthcare Systems, Mechanisms and Paediatric Applications. Healthcare (Basel) [Internet]. 2024;12(15):1-26. doi: https://doi.org/10.3390/healthcare12151547
34. Hailemeskel B, Ziregbe E, Tran CF, Pharmd SBW, Ahari-Lahagh A, Kumarra S, et al. Complementary and Alternative Medicine (CAM) Utilization by Howard University (HU) First Year Pharmacy Students: Survey and Review of Most Commonly Used Herbs. Curr Res Integr Med [Internet]. 2017;2(3):37-41. Available from: https://www.pulsus.com/abstract/complementary-and-alternative-medicine-cam-utilization-by-howard-university-hu-first-year-pharmacy-students-survey-andrn-3980.html
35. Yates JS, Mustian KM, Morrow GR, Gillies LJ, Padmanaban D, Atkins JN, et al. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer [Internet]. 2005;13(10):806-11. doi: https://doi.org/10.1007/s00520-004-0770-7
36. Marshik PL, Kharat AA, Jakeman B, Borrego ME, Dodd MA, Bachyrycz A, et al. Complementary and Alternative Medicine and Therapy Use in a Diverse New Mexican Population. J Altern Complement Med [Internet]. 2016;22(1):45-51. doi: https://doi.org/10.1089/acm.2014.0378
37. Nissen NK. Challenging perspectives: Women, complementary and alternative medicine, and social change. Interface [Internet]. 2011;3(2):187-212. Available from: http://www.interfacejournal.net/wordpress/wp-content/uploads/2011/12/Interface-3-2-Nissen.pdf
38. Alwhaibi M, AlRuthia Y, Meraya AM. Gender Differences in the Prevalence of Complementary and Alternative Medicine Utilization among Adults with Arthritis in the United States. Evid Based Complement Alternat Med [Internet]. 2019:8739170. doi: https://doi.org/10.1155/2019/8739170
39. Bari S, Chineke I, Darwin A, Umar A, Jim H, Muzaffar J, et al. Awareness, Use and Outlook of Complementary and Alternative Medicine (CAM) Options in an Underserved, Uninsured Minority Cancer Patient Population. Integr Cancer Ther [Internet]. 2021;20: 15347354211051622. doi: https://doi.org/10.1177/15347354211051622
40. Dufter SA, Hübner J, Ahmadi E, Zomorodbakhsch B. Traits of cancer patients and CAM usage. J Cancer Res Clin Oncol [Internet]. 2021;147(12):3685-92. doi: https://doi.org/10.1007/s00432-021-03605-7
41. Kim K, Kim SH, Ok ON, Kim IR, Lee S, Kim SH, et al. Use of complementary and alternative medicine by lymphoma survivors in South Korea. Eur J Oncol Nurs [Internet]. 2018;33:91-6. doi: https://doi.org/10.1016/j.ejon.2018.01.012
42. Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal Remedies in the United States: Potential Adverse Interactions With Anticancer Agents. J Clin Oncol [Internet]. 2004;22(12):2489-503. doi: https://doi.org/10.1200/JCO.2004.08.182
43. Gavamukulya Y, Wamunyokoli F, El-Shemy HA. Annona muricata: Is the natural therapy to most disease conditions including cancer growing in our backyard? A systematic review of its research history and future prospects. Asian Pac J Trop Med [Internet]. 2017;10(9):835-48. doi: https://doi.org/10.1016/j.apjtm.2017.08.009
44. Gu I, Brownmiller C, Howard L, Lee SO. Chemical Composition of Volatile Extracts From Blackberries, Black Raspberries, and Blueberries and Their Apoptotic Effect on A549 Non-Small-Cell Lung Cancer Cells. Curr Dev Nutr [Internet]. 2022;6(Suppl 1):284. doi: https://doi.org/10.1093/cdn/nzac053.025
45. Hoshyar R, Mahboob Z, Zarban A. The antioxidant and chemical properties of Berberis vulgaris and its cytotoxic effect on human breast carcinoma cells. Cytotechnology [Internet]. 2016;68(4):1207-13. doi: https://doi.org/10.1007/s10616-015-9880-y
46. Boon H, Stewart M, Kennard MA, Gray R, Sawka C, Brown JB, et al. Use of Complementary/Alternative Medicine by Breast Cancer Survivors in Ontario: Prevalence and Perceptions. J Clin Oncol [Internet]. 2000;18(13):2515-21. doi: https://doi.org/10.1200/JCO.2000.18.13.2515
47. Martínez-Ramos N, Mariño C, Olaya-SanMiguel LC, Murillo R. (2024). Use of Alternative Medicine in Cancer Patients in the City of Neiva (Colombia). Univ Med [Internet]. 2024;65. doi: https://doi.org/10.11144/Javeriana.umed65.umap
48. Asiimwe JB, Nagendrappa PB, Atukunda EC, Kamatenesi MM, Nambozi G, Tolo CU, et al. Prevalence of the Use of Herbal Medicines among Patients with Cancer: A Systematic Review and Meta-Analysis. Evid-Based Complement Alternat Med [Internet]. 2021: 9963038. doi: https://doi.org/10.1155/2021/9963038
Supplementary material
Supplementary Table 1. Percentage Distribution of Cancer Diagnosis.
| ICD-10 Code | Type of Cancer | % |
|---|---|---|
| C50 | Breast Cancer | 31.4 |
| C18 | Colon Cancer | 9.3 |
| C16 | Stomach Cancer | 6.8 |
| C56 | Ovarian Cancer | 5.9 |
| C53 | Cervical Cancer | 4.9 |
| D | Neoplasms | 3.4 |
| C61 | Prostate Cancer | 3.2 |
| C34 | Bronchus and Lung Cancer | 2.5 |
| C82 | Follicular Lymphoma | 2.1 |
| C15 | Esophageal Cancer | 2.1 |
| C54 | Uterine Body Cancer | 1.9 |
| Other | 26.5 |
Supplementary Table 2. 15 Most Common CAM.
| CAM | n | Intention of Use | |||
|---|---|---|---|---|---|
| Curative | Palliative | Both | Other | ||
| Products Based on Soursop | 126 | 44 | 39 | 16 | 37 |
| Soursop | 110 | 36 | 28 | 12 | 34 |
| Soursop Leaf | 26 | 8 | 11 | 4 | 3 |
| Cranberry | 108 | 26 | 46 | 7 | 29 |
| Transfer Factors | 94 | 33 | 27 | 11 | 23 |
| Blackberry | 91 | 19 | 34 | 3 | 35 |
| Spinach | 86 | 22 | 31 | 7 | 26 |
| Grapes | 83 | 21 | 31 | 6 | 25 |
| Chicken Feet | 81 | 23 | 12 | 4 | 42 |
| Anamu (Guinea Hen Weed) | 60 | 19 | 20 | 5 | 16 |
| Blueberry | 46 | 11 | 13 | 3 | 19 |
| Beetroot | 36 | 6 | 19 | 2 | 9 |
| Lentils | 30 | 8 | 5 | 3 | 14 |
| Celery | 25 | 4 | 16 | 2 | 3 |
| Carrot | 25 | 4 | 13 | 4 | 4 |
| Restriction of Red Meats | 22 | 11 | 2 | 5 | 4 |