IgA, IgM, and IgG Antibodies and Neutralizing Activity against SARS-CoV-2 in a Population of Colombian Healthcare Workers and Hospital Staff
Anticuerpos IgA, IgM e IgG y actividad neutralizante frente al SARS-CoV-2 en una población de trabajadores de la salud y personal hospitalario colombiano
Norma Cecilia Serrano Díaz, Claudia Carolina Colmenares-Mejía, Doris Cristina Quintero-Lesmes , Isail Salazar Acosta, Diana Paola Suárez Suárez, Angie Yarlady Serrano-García, Olga Lucía Sopó-Rincón, Ligia Stella Meneses Duarte, Bladimiro Rincón Orozco
Abstract
Introduction. During viral infections, the body produces binding antibodies (n-NAbs) and neutralizing antibodies (NAbs). NAbs prevent viral infection of host cells. Since COVID-19's onset in 2020, vaccine research has focused on eliciting high NAb levels.
Objective. To evaluate IgA, IgM, and IgG levels in Colombian healthcare workers and compare NAb test results from DIA.PRO and CPAS.
Method. A cross-sectional study in Bucaramanga, Colombia, collected samples from healthcare workers in June-July and November 2021. Data was gathered via an online survey and blood samples. Antibody levels were measured with AESKULISA® kits, and neutralizing activity was assessed using DIA.PRO and cPass kits. Statistical analyses used Wilcoxon tests and Pearson correlation.
Results. Eighty participants were initially assessed, with forty-seven re-evaluated. Most were women who had received the BioNTech-Pfizer vaccine. Antibody levels declined over time; IgA and IgM were lower in the second sampling, while IgG remained high. Prior COVID-19 infection correlated with higher antibody levels. DIA.PRO and CPAS tests showed strong agreement, with excellent neutralizing reactivity in most participants.
Conclusion. Neutralizing antibody levels against SARS-CoV-2 decrease but remain effective in most healthcare workers, supporting BNT162b2 vaccine efficacy. Further research is needed on emerging variants and neutralizing antibodies in COVID-19 management.
Keywords
Neutralizing antibodies; coronavirus infections; vaccine; occupational health.
Resumen
Introducción. Durante las infecciones virales, el cuerpo produce anticuerpos de unión (n-NAbs) y anticuerpos neutralizantes (NAbs). Los NAbs previenen la infección viral de las células del huésped. Desde el inicio de la COVID-19 en 2020, la investigación sobre vacunas se ha centrado en inducir altos niveles de NAbs.
Objetivo. Evaluar los niveles de IgA, IgM e IgG en trabajadores de la salud colombianos y comparar los resultados de las pruebas de NAbs de DIA.PRO y CPAS.
Método. Se realizó un estudio transversal en Bucaramanga, Colombia, en el que se recolectaron muestras de trabajadores de la salud entre junio-julio y noviembre de 2021. Los datos se obtuvieron a través de una encuesta en línea y muestras de sangre. Los niveles de anticuerpos se midieron con kits AESKULISA® y la actividad neutralizante se evaluó utilizando los kits DIA.PRO y cPass. Los análisis estadísticos se realizaron con pruebas de Wilcoxon y correlación de Pearson.
Resultados. Ochenta participantes fueron evaluados inicialmente, y cuarenta y siete fueron reevaluados. La mayoría eran mujeres que habían recibido la vacuna BioNTech-Pfizer. Los niveles de anticuerpos disminuyeron con el tiempo; IgA e IgM fueron más bajos en el segundo muestreo, mientras que IgG se mantuvo elevado. La infección previa por COVID-19 se correlacionó con niveles más altos de anticuerpos. Las pruebas de DIA.PRO y CPAS mostraron una fuerte concordancia, con excelente reactividad neutralizante en la mayoría de los participantes.
Conclusión. Los niveles de anticuerpos neutralizantes contra el SARS-CoV-2 disminuyen, pero se mantienen efectivos en la mayoría de los trabajadores de la salud, lo que respalda la eficacia de la vacuna BNT162b2. Se necesita más investigación sobre las variantes emergentes y los anticuerpos neutralizantes en el manejo de la COVID-19.
Palabras clave
Anticuerpos neutralizantes; infecciones por coronavirus; vacuna; salud ocupacional.
Introduction
The epidemiological situation of COVID-19 in Colombia, as reported by the National Institute of Health (INS), shows a sustained decline in new infections throughout 2024. As of epidemiological week 22, 6,407,261 confirmed cases have been reported since the pandemic's beginning, with an incidence rate of 12,158.92 per 100,000 inhabitants. Additionally, 143,299 deaths have been recorded, representing a mortality rate of 271.93 per 100,000 inhabitants and a cumulative case-fatality rate of 2.23%. Since the second week of July 2021, there has been a consistent decrease in the magnitude of infection peaks, with a significant impact observed during the spread of the Omicron variant in late 2021 and early 2022. These epidemiological data underscore the importance of this study, which analyzes the dynamics of the immune response in healthcare workers, a critical population in managing the pandemic. This analysis complements the national pandemic overview and provides valuable insights for optimizing vaccination strategies and epidemiological surveillance in the country [1].
Two types of antibody responses occur in the body during an infection: the development of binding antibodies (n-NAbs) and neutralizing antibodies (NAbs). Unlike n-NAbs, neutralizing antibodies bind to the virion and neutralize it. Specifically, neutralizing antibodies inhibit the virus’s ability to infect without requiring the involvement of other immune cells [2]. Since 2020, the COVID-19 pandemic has prompted extensive research to develop effective vaccines that induce robust immunological memory and adequate levels of neutralizing antibodies [3]. The SARS-CoV-2 virus has different proteins in its structure. The Spike protein is one of current interest since it contains a Receptor-Binding Domain (RBD) in the S1 subunit, on which neutralizing antibodies act upon blocking the binding of RBD to the host Angiotensin-Converting Enzyme 2 (ACE2), thus preventing viral proliferation [4].
The neutralizing humoral response can be observed in up to 96.5% of individuals after 14 days of one dose, two doses, or boosters against SARS-CoV-2, but these values can be variable, depending on the cohort studied [5]. Immunoglobulin M (IgM) is one of the first antibodies to show activity, being detectable since day four and until approximately day 30 when it begins to decrease. Nevertheless, it has also been observed detectable levels of Immunoglobulin G (IgG) and Immunoglobulin A (IgA) synergistically increase together with IgM or days before its finding [6,7]. In general, neutralizing activity can be variable depending on factors such as viral load and medical history. Still, it is possible to detect neutralizing activity between 6 and 15 days after vaccination, with a half-life of 26 days. Neutralizing antibody levels may be low or absent approximately after 6-8 months [7,8]. In addition, SARS-CoV-2 variants can influence infection or vaccine-induced neutralizing activity [9,10].
The SARS-CoV-2 mutation rate is slower than other types of RNA viruses (1×10−3 nucleotide substitutions each year) [11]. However, there are currently variants of concern like the Omicron variant (lineages B.1.1 .529, BA.1, BA.1.1, BA.2, BA.3, BA.4, and BA.5) that can alter the efficacy of vaccination and decrease the response of neutralizing antibodies [12,13].
Although recent research has focused on the neutralizing activity of antibodies against the Spike protein in healthcare workers [5,12], the duration and efficacy of neutralizing humoral immunity induced after vaccination remain uncertain. Understanding the antibody response is crucial to supporting therapeutic efforts and epidemiological surveillance.
Therefore, this research aimed to: 1) analyze changes in antibody levels (IgA, IgM, IgG) in a Colombian population of health workers and hospital staff; 2) assess neutralizing antibody functionality at a specific time point; and 3) evaluate concordance between DIA.PRO and CPAS tests for neutralizing reactivity.
Method
Design and population
A cross-sectional observational study (with two separate samplings) was carried out in the Metropolitan Area of Bucaramanga (Santander, Colombia). Healthcare and front-line workers from two health institutions were invited to participate with the vaccination schedule for COVID-19 (two doses with BNT162b2 BioNTech-Pfizer [Messenger RNA])
Sampling and sample
A convenience sampling was selected. Recruitment took place between June and July (first sampling) and November (second sampling) 2021.
Data collection and variables
All participants self-completed an online survey on socio-demographic data (age, ethnicity, marital status, education level, socioeconomic level, residence municipality), occupation, cigarette smoking status, medical history, COVID-19-related symptoms in the last two weeks, history of COVID-19 confirmed infection by PCR, antibodies tests previously performed (IgM / IgG), type of transportation used to go to work, contact with people (at home or to the workplace with suspected or confirmed COVID-19 infection, and vaccination scheme to date (including doses and side effects).
Study data were collected (including e-consent) and managed using REDCap [14] electronic data capture tools hosted at Fundación Cardiovascular de Colombia.
The tests used to establish COVID-19 infection history (confirmed by RT-PCR) were described and can be consulted in previously published articles [15,16].
IgG, IgM, IgA measurement - infection immune response
5 mL of peripheral blood sample was obtained from each participant in each assessment. Samples that did not meet the manufacturer's quality requirements were excluded (icteric, lipemic, hemolytic, or bacterial contamination). For IgG / IgM / IgA antibodies detection against SARS-CoV-2 virus, qualitative and quantitative test kits AESKULISA® SARS-CoV-2 S1 NP IgA, IgG, and IgM were used [17]. This kit detects antibodies specific to the S1 domain of the glycosylated Spike protein of SARS-CoV-2. AESKULISA® immunoassays have a sensitivity of >95% and a specificity of >99%. The detection limit is the lowest analyte concentration detectable by this method, while the quantification limit is the lowest concentration measurable with specified precision and accuracy. According to the supplier's quality control certificate, the detection limit range is 8-12 U/ml and the measurement range is 3-100 U/ml. Samples below the detection limit range are considered negative, while those above are considered positive. Therefore, the detection limit is used to determine the presence or absence of antibodies in the sample.
Neutralizing Antibodies measurement - vaccine induces a response
For detection of neutralizing activity, ACE2-RBD Neutralization test from DIA.PRO Diagnostics Bioprobes [18] was used, which in addition to determining the presence of total antibodies against RBD, measures the real biological activity of antibodies by inhibiting the binding of RBD from SARS-CoV -2 to its ACE2 receptor, preventing the virus from entering the target cells. It is a surrogate Virus Neutralizing Test (sVNT), a method that mimics virus-host interaction in one well of an ELISA plate. It uses SARS-CoV-2-specific recombinant glycosylated RBD instead of a living virus, providing a safer solution as cells, biosafety containment facilities, and highly trained operators are not required.
The limit ranges were those established by the supplier in the quality control certificate: detection limit range 8-12 U / ml and measurement range 3-100 U / ml. The evaluation of a sample below the limit range was established as negative and above positive.
Neutralizing Antibodies CPAS measurement
The determination of neutralizing antibodies against SARS-CoV-2 was performed using the cPass SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript, USA, L00847). Serum samples and controls were diluted and incubated with the variant-specific RBD-HRP conjugate. After incubation, the reaction was transferred to a 96-well microtiter plate coated with ACE2. The microtiter plate was then washed, followed by the enzyme/substrate interaction with the addition of TMB and stopping the reaction. The optical density at 450 nm was measured using a plate reader (Labtech, Germany), and the detection of neutralizing antibodies was determined based on a signal inhibition of 30% or greater.
Reactivity for vaccine-induced response
The assay procedure has to be applied specifically for the determination of neutralizing antibodies developed upon vaccination with a vaccine able to stimulate the production of IgG (or IgG+IgA) to the Receptor Binding Domain (or RBD) of SARS-CoV-2 Spike 1 antigen. This method will rule out samples whose titer of neutralizing antibodies is below 1:10 and point out those vaccinated individuals for which the vaccine has stimulated a good or excellent titer of antibodies capable of preventing RBD from binding to ACE2 and therefore the development of the infection. After reading the mean Optic Density OD450nm of the negative control and the OD 450nm /620-630nm of the samples, the Percentage of Neutralization of the sample (NS%) was calculated following a mathematical formula that allowed us to classify patients according to the following interpretations parameters: 1. Low or not reactive: NS% <20% (<10WHO IU/mL). 2. Moderate neutralizing reactivity: NS% 20 - <30% (10-100WHO IU/mL). 3. Good neutralizing reactivity: NS% 30 - <60% (100-400WHO IU/mL). 4. Excellent neutralizing reactivity: NS% 60 - <100% (>400WHO IU/mL). Reactivity was assessed for all participants in the first sampling.
Virus variant identification
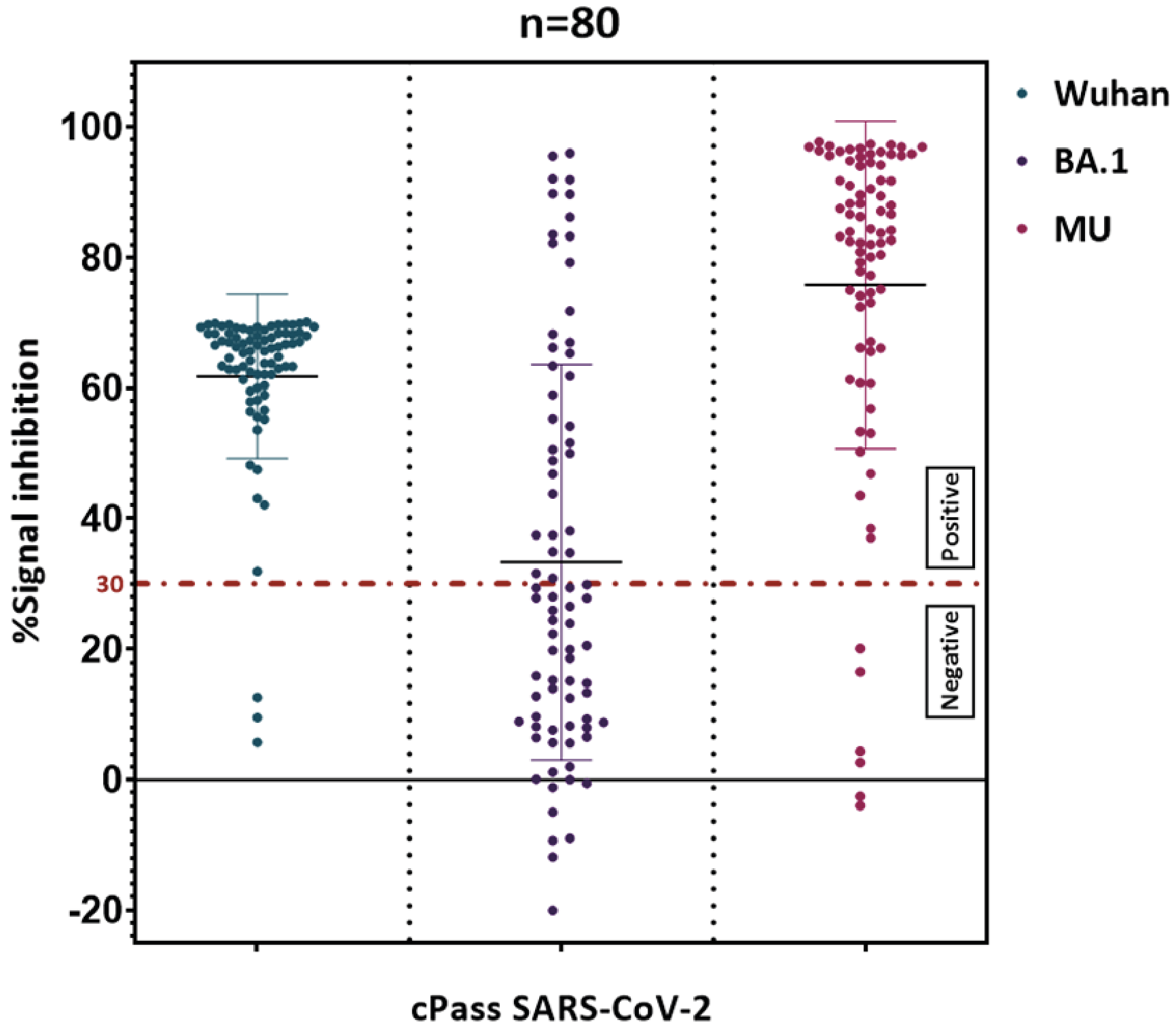
During that period, several SARS-CoV-2 variants were in circulation, including the original Wuhan strain, the Omicron variant BA.1, and the MU variant. The Wuhan strain, first identified in Wuhan, China, represents the initial form of the virus with its original genetic sequence. The Omicron variant BA.1 emerged later, characterized by its high transmissibility and numerous mutations in the spike protein, which allowed it to partially evade immune responses and quickly become a dominant strain. The MU variant, although less widespread, possessed unique mutations that raised concerns about potential resistance to neutralizing antibodies and vaccines.
Delta times
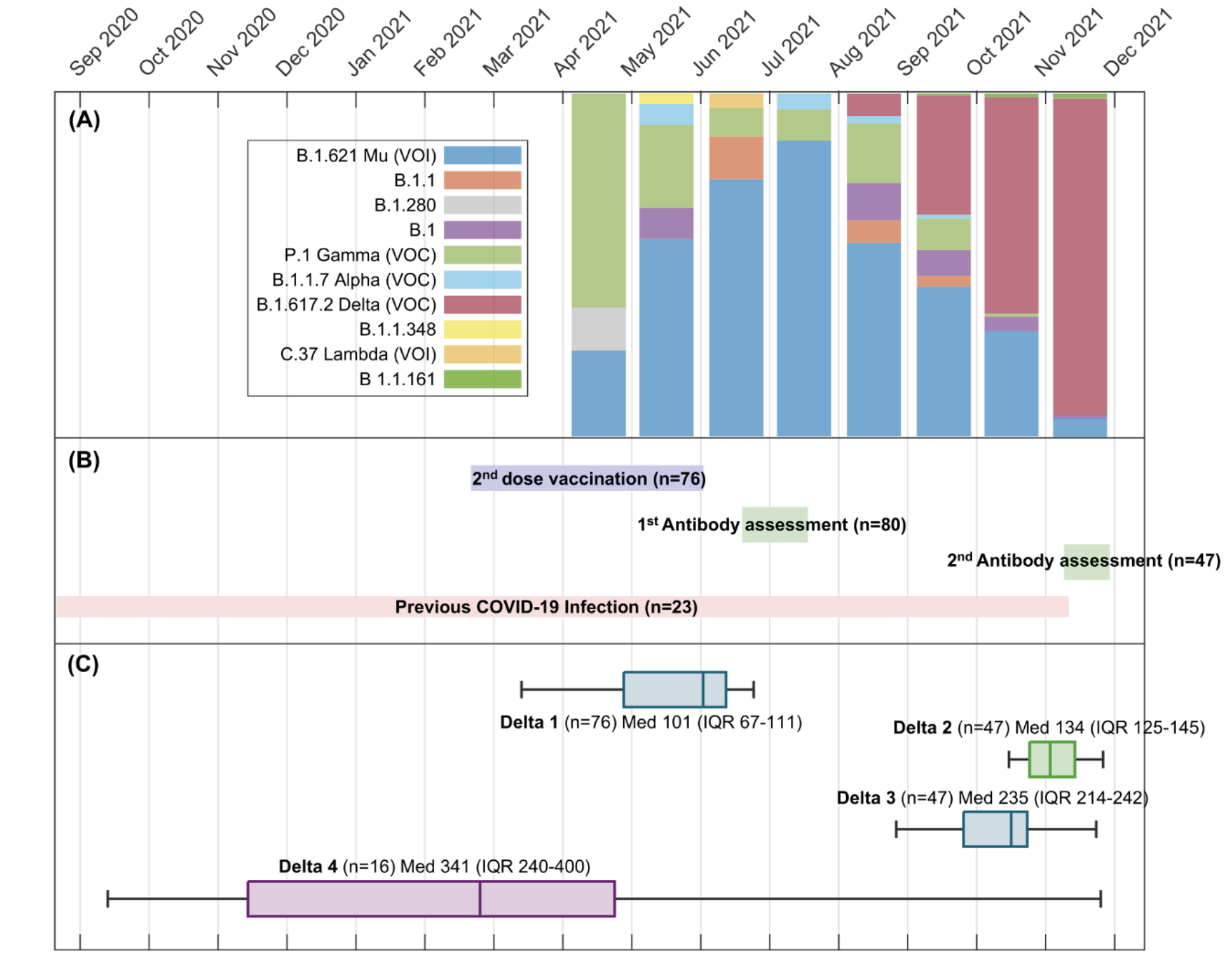
Change in antibody levels was assessed through several time intervals [in days] (Figure 1) defined as Delta 1: Second dose date and study entry date; Delta 2: Study entry date and second sample collection date; Delta 3: Second dose date and second sample collection date; Delta 4: previous COVID-19 infection and second sample collection date.
Figure 1. Participants recruitment and antibody assessment.
A. SARS-CoV-2 variants in Bucaramanga and metropolitan area during participant recruitment.
B. Dates for vaccine second dose against SARS-CoV-2 and recruitment period
C. Delta 1: Second vaccine dose date and study entry date; Delta 2: Study entry date and second sample collection date; Delta 3: Second dose date and second sample collection date; Delta 4: previous COVID-19 infection and second sample collection date.
Statistical methods
Numerical variables are reported as means and standard deviation or median with Interquartile Range (IQR) according to their distribution, and absolute and relative frequencies for qualitative data. Distribution was assessed through Shapiro wilks test. Differences between first and second measurement levels were established by Wilcoxon signed-rank test and the correlation between DIA.PRO test and CPASS test for reactivity were calculated with Pearson correlation. For individuals who were included in both assessments, a comparison was done between those participants with the highest antibody levels (≥10.000 U/mL) in the two assessments and those who did not, through the chi-squared test or ANOVA for qualitative and quantitative variables, respectively. A p-value <0.05 was interpreted as statistically significant for hypothesis testing. Statistical analysis was done in Stata 15.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Fundación Cardiovascular de Colombia (protocol code CEI-2020-01858, December 20th, 2020) under the name of “Fortalecimiento de capacidades en Ciencia y Tecnología del Laboratorio de Biología Molecular de la Fundación Cardiovascular de Colombia para atender problemáticas asociadas con agentes biológicos de alto riesgo para la salud humana Bucaramanga/ Santander”, project from which articles such as the one presented here have been derived.
Results
80 participants were included in first assessment, and 47 in the second one. Most of them were women, working as attending physicians or nurses in comprehensive care (ambulatory services, hospitalization, emergency room, and laboratory), followed by Intensive Care, and COVID areas (Table 1). On the other hand, Figure 1 shows the time of recruitment of participants, as well as their distribution of possible strains and antibody assessment.
Table 1. Sociodemographic characteristics in first and second assessment.
| Variable | 1st assessment | 2nd assessment |
|---|---|---|
| Age, years | 36.1 (8.9) * | 37 (9.1)* |
| Sex | ||
| Female | 65 (81.2) | 39 (83.0) |
| Male | 15 (18.8) | 8 (17.0) |
| Civil status | ||
| Married | 42 (52.5) | 23 (48.9) |
| Divorced/widower | 3 (3.7) | 3 (6.3) |
| Single | 35 (43.7) | 21 (44.7) |
| Municipality | ||
| Bucaramanga | 29 (36.3) | 19 (40.4) |
| Floridablanca | 40 (50.0) | 23 (48.9) |
| Girón | 2 (2.5) | |
| Piedecuesta | 9 (11.2) | 5 (10.6) |
| Socioeconomic status | ||
| Low (1 and 2) | 15 (19.0) | 9 (19.1) |
| Medium (3 and 4) | 44 (55.7) | 27 (57.4) |
| High (5 and 6) | 20 (25.3) | 11 (23.3) |
| Number of people that live with | 3 (2 - 4)** | 3 (2 - 4)** |
| Scholarity | ||
| Technician | 25 (31.3) | 13 (27.7) |
| Graduate | 24 (30.0) | 15 (31.9) |
| Post-graduate | 31 (38.7) | 19 (40.4) |
| Occupation | ||
| Auxiliary nurse | 21 (26.3) | 9 (19.1) |
| Laboratory technician | 1 (1.3) | 1 (2.1) |
| Laboratory staff | 10 (12.5) | 5 (10.6) |
| Administrative nurse | 3 (3.7) | 2 (4.3) |
| Assistant nurse | 14 (17.5) | 11 (23.4) |
| Administrative physician | 1 (1.3) | 1 (2.1) |
| Attending Physician | 16 (20.0) | 9 (19.1) |
| Assistant physician | 12 (15.0) | 8 (17.0) |
| Administrative Staff | 2 (2.5) | 2 (4.3) |
| COVID Hospital | 35 (43.7) | 19 (40.4) |
| Non-COVID Hospital | 45 (56.3) | 28 (59.6) |
| Working service | ||
| Comprehensive care | 32 (40.0) | 19 (40.4) |
| Administrative | 9 (11.3) | 6 (12.8) |
| ICU | 23 (28.8) | 13 (27.7) |
| COVID areas | 16 (20.0) | 9 (19.1) |
Notes. *Mean (SD). Socioeconomic status: 1 lowest, 6 highest. Occupation: Auxiliar nurse is a technician degree. **Median (IQR). Comprehensive care includes ambulatory services, hospitalization, emergency room, and laboratory. COVID areas include hospitalization, emergency room and ICU.
Smoking or presence of positive medical history was low. Regarding previous exposure to COVID, it was frequent to report previous positive PCR for SARS-CoV-2 by the second assessment meanwhile positive antibody (IgG or IgM) tests were similar in both assessments (Table 2). Most of the participants (96.3%) had received BioNTech-Pfizer vaccine by first sampling.
Table 2. Participants medical history in first and second assessment.
| Variable | 1st assessment | 2nd assessment |
|---|---|---|
| Smoking | ||
| Never smoked | 58 (72.5) | 36 (76.6) |
| No, but people around me do | 1 (1.2) | - |
| Yes, but not currently | 18 (22.5) | 10 (21.3) |
| Yes | 3 (3.8) | 1 (2.1) |
| Medical history | ||
| None | 61 (76.2) | 38 (80.9) |
| Obesity | 7 (8.8) | 6 (12.8) |
| COPD/Asthma | 5 (6.2) | - |
| Hypertension | 3 (3.8) | 1 (2.1) |
| Hypothyroidism | 4 (5.0) | 2 (4.2) |
| Symptoms during the last two weeks | ||
| None | 60 (75.0) | 34 (72.3) |
| Headache | 6 (7.5) | 4 (8.5) |
| Flu alike symptoms | 14 (17.5) | 9 (19.2) |
| Previous PCR Tests | 4 (3 - 4)* | 4 (3 - 4)* |
| Previous antibody assessments | 3 (3 - 4)* | 3 (2 - 4)* |
| Previous COVID infection** | 22 (27.5) | 15 (31.9) |
| Previous positive antibody (IgG or IgM) test | 33 (41.2) | 19 (40.4) |
Notes. *Median (IQR). ** Previous COVID infection confirmed by PCR.
Total antibody levels vaccine-induced
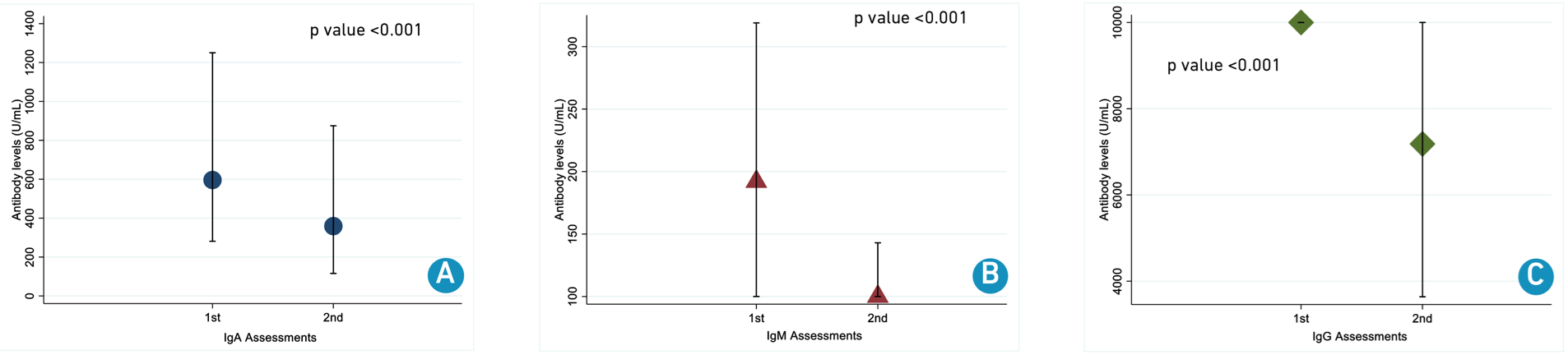
Total antibody median levels for first sampling were: IgA 596 U/mL (IQR 281 - 1,250); IgM 192 U/mL (IQR <100 - 319); IgG >10,000 U/mL (IQR <10,000 - >10,000) and for second sampling were IgA 359 U/mL (IQR 116 - 875); IgM <100 U/mL (IQR <100 - 143); IgG 7,183 U/mL (IQR 3,637 - >10,000). In general, antibody levels statistically decreased over time (Figure 2).
Figure 2. Neutralizing antibody levels between two measurements.
A. Immunoglobulin A; B. Immunoglobulin M; C. Immunoglobulin G.
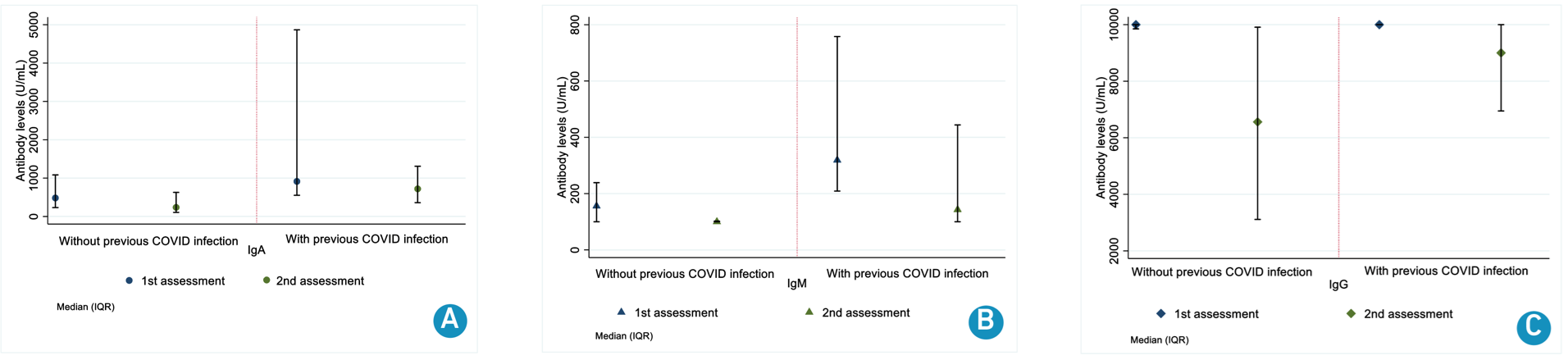
Median levels were higher for participants with previous COVID infection in first [IgA 914 U/mL (IQR 552 - 4,867); IgM 319 U/mL (IQR 209 - 758); IgG 10,000 U/mL (IQR 10,000 - 10,000) vs no COVID history IgA 482 U/mL (IQR 231 - 1,083); IgM 156 U/mL (IQR 100 - 239); IgG 10,000 U/mL (IQR 9,849 - 10,000)] and second [IgA 720 U/mL (IQR 359 - 1,309); IgM 143 U/mL (IQR 100 - 444); IgG 9,001 U/mL (IQR 6,947 - 10,000) vs IgA 237 U/mL (IQR 105 - 628); IgM 100 U/mL (IQR 100 - 102); IgG 6,560 U/mL (IQR 3,112 - 9,909)] (Figure 3).
Figure 3. Antibody levels in both assessments according to history of COVID infection.
A. Immunoglobulin A; B. Immunoglobulin M; C. Immunoglobulin G.
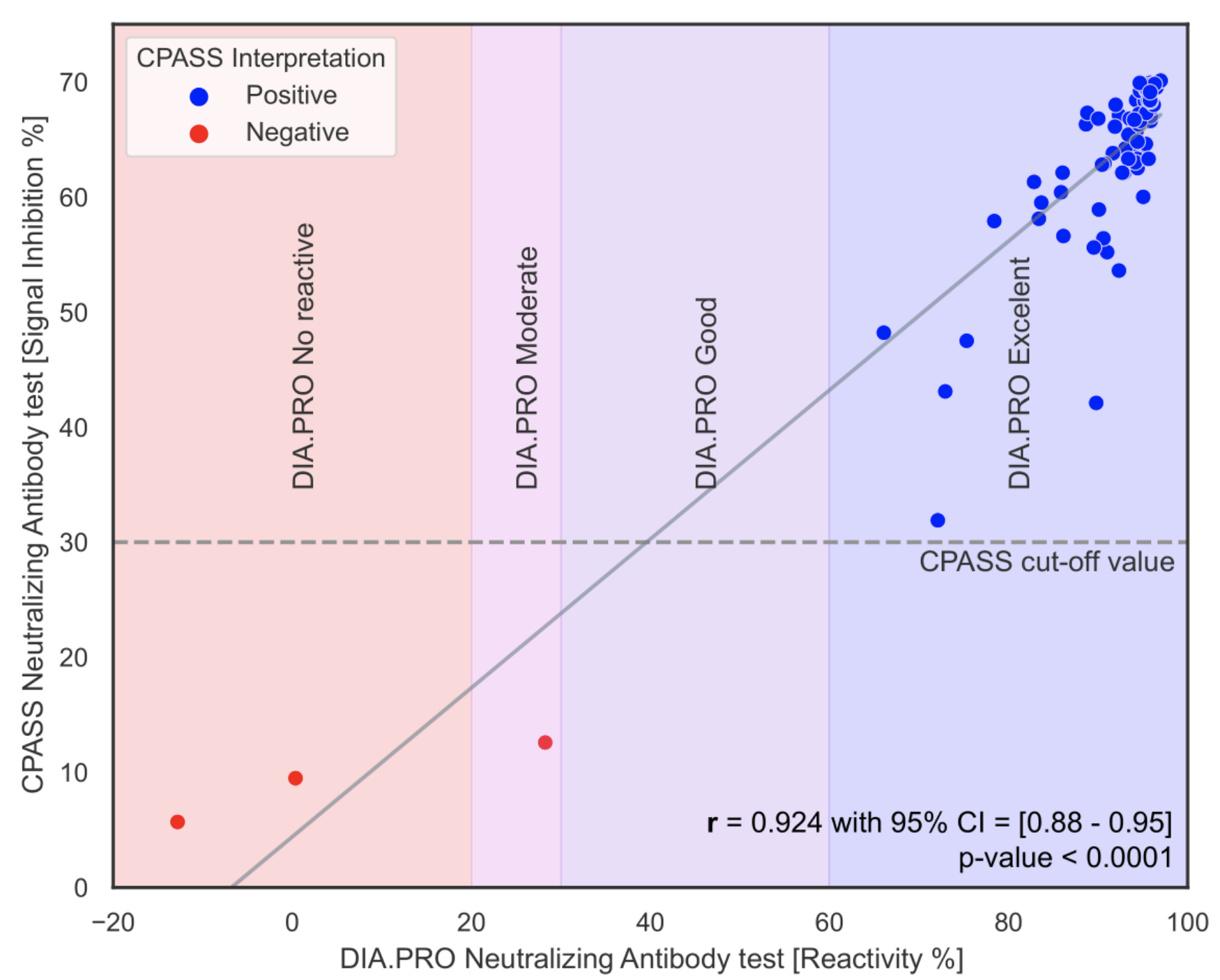
Figure 4 shows the correlation between the CPASS assay and the DIA.PRO reactivity test. This figure demonstrates the relationship between the results obtained from both methods, indicating a high level of agreement in detecting SARS-CoV-2 neutralizing antibodies.
Figure 4. Correlation between CPASS and DIA.PRO reactivity test.
Reactivity
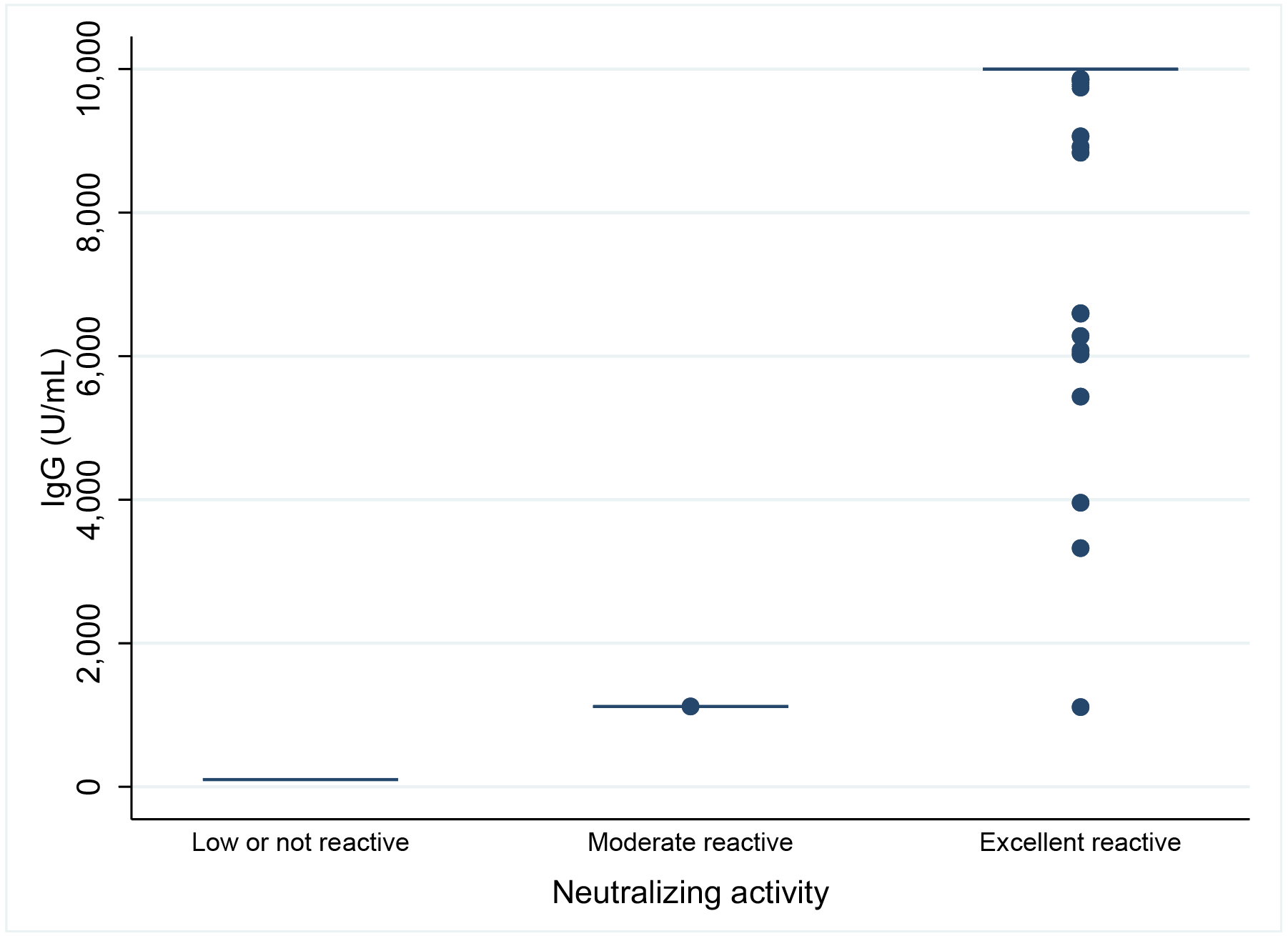
Regarding antibody reactivity, two participants (2.5%) were low or non-reactive with percentage reactivity of 0.36225 and -12.80795; one participant (1.25%) had moderate reactivity and remain participants had excellent reactivity (n=77, 96.25%). Agreement between antibody levels and neutralizing activity is shown in supplementary material Figure S1.
Participants with low or not reactive activity reported not having been vaccinated at the moment of first sampling are shown in supplementary material Figure S2.
Supplementary material (Figure S3) shows the detection of SARS-CoV-2 neutralizing antibodies using the CPASS assay. This figure provides detailed results of the antibody detection process, highlighting the assay's effectiveness in identifying neutralizing antibodies in different variants.
Additionally, some characteristics were evaluated in participants with the highest IgG levels for both assessments in comparison with those with lower levels. Although there was a higher frequency of previous COVID infection in patients with higher IgG levels in the first and second sampling, it was not statistically significant. There were also no differences in time deltas between the two groups (Table 3).
Table 3. Characteristics evaluated according IgG levels in first and second assessment.
| Variable | IgG >10.000 U/mL in both samplings | ||
|---|---|---|---|
| Yes | No | p value *** | |
| Delta 3* (days) | 241 (228 - 250.5) | 233 (213 - 242) | 0.092 |
| Delta 4* (days) | n=4 412 (357 - 453) |
n=9 213 (195 - 300) |
0.643 |
| Age* (years) | 40 (31 - 49) | 37 (30 - 41) | 0.217 |
| Smoking | |||
| Never smoked | 9 (75.0) | 27 (77.1) | 0.781 |
| Yes, but not currently | 3 (25.0) | 7 (20.0) | |
| Yes | - | 1 (2.9) | |
| Medical history | |||
| Yes | 3 (25.0) | 6 (17.1) | 0.674 |
| Previous COVID infection** | 5 (41.7) | 10 (28.6) | 0.401 |
| Previous positive antibodies (IgG or IgM) | 4 (33.3) | 15 (42.9) | 0.562 |
| Working service | |||
| Comprehensive care | 6 (50.0) | 13 (37.1) | 0.658 |
| Administrative | 1 (8.3) | 5 (14.3) | |
| ICU | 2 (16.7) | 11 (31.4) | |
| COVID areas | 3 (25.0) | 6 (17.1) | |
Notes. *Median (IQR) for quantitative variables. **Previous COVID infection confirmed by PCR. ***Shapiro-Wilk test
Discussion
Measurements of antibody levels and their reactivity were conducted on healthcare workers or frontline staff (hospital personnel). It was found that although neutralizing antibody levels remained detectable at the time of the second evaluation, there was a decrease in the levels, especially in immunoglobulin M. This decrease may be because it is the first immunoglobulin to show reduced anti-RBD activity, followed by IgG and IgA, as previously reported in a study conducted by Gaebler et al., which involved a cohort of 87 participants. The study showed a 53% decrease in anti-RBD IgM, followed by a 32% decrease in IgG and a 15% decrease in IgA between 1.3 and 6.2 months after SARS-CoV-2 infection [19].
Likewise, the relationship between time variables, comorbidities, previous COVID-19 infection, and IgG levels (>10,000 U/mL) in both measurements was studied, due to a longer-lasting immunity associated with memory B cells [20]. However, no statistically significant differences were found
Regarding reactivity, IgG showed a significant change between the first and second evaluations, with the latter showing more optimal neutralizing activity against SARS-CoV-2, likely enhanced by prior COVID-19 infection. The lack of reactivity (<20% neutralization) for IgG in 2 participants during the first evaluation could be explained by the fact that this immunoglobulin had not yet reached its peak activity at that time or because the SARS-CoV-2 infection was mild. It has been found that IgG response is more robust in patients who had a severe case of COVID-19 [7].
According to published literature, vaccines such as BBIBP-CorV, AZD1222, and BNT162b2 have shown a higher level of neutralizing antibodies after vaccination against SARS-CoV-2 compared to other vaccines [21]. In this study, the majority of participants (96.3%) were immunized with BioNTech-Pfizer (BNT162b2), which could explain the high levels of neutralizing antibodies. However, it was observed that antibody titers decreased over time, with approximately 4 months between the first and second sample collections, consistent with findings in another study [8].
The decrease in the durability of antibody levels may be influenced by the natural course of humoral immunity [7]. Likewise, the emergence of new variants of concern, including the currently circulating Omicron variant and its lineages (B.1.1.529, BA.1, BA.1.1, BA.2, BA.3, BA.4, and BA.5) [11], due to its high mutational capacity (up to 30 mutations in the Spike protein and 15 in the receptor-binding domain [12], may grant SARS-CoV-2 the ability to evade the humoral immunity acquired after COVID-19 or vaccination [22]. This is corroborated by a study conducted in a population similar to ours, which found that 2 months after receiving two doses of BNT162b2, participants had neutralizing antibody titers 11.8 times lower against the Omicron variant compared to the D614G variant [12].
Commercial kit types can influence the detection of neutralizing antibodies: serological assays such as Roche (detects total antibodies against the nucleocapsid), Abbott (detects IgG against the nucleocapsid), and EUROIMMUN (detects IgG against the S1 domain) [23] are not specifically directed at the regions that predict higher neutralizing efficacy, such as the S1, S2, and RBD domains of the COVID-19 Spike protein [6] (except EUROIMMUN). In this study, the DIA.PRO neutralization assay was used, which detects the total antibodies produced against the RBD antigen of SARS-CoV-2 and the biological activity of these immunoglobulins by preventing the binding of RBD to the host ACE2 receptor. This surrogate Virus Neutralizing Test (sVNT) has a sensitivity and specificity of 100% [18], which would provide reliability to the results obtained through this research.
This study´s analysis of different immunoglobulin classes (IgA, IgM, and IgG) highlights distinct patterns in the immune response to SARS-CoV-2. Specifically, IgA and IgM have been shown to play critical roles in viral neutralization during the early stages of infection. Previous studies suggest that IgA, predominant in mucosal surfaces, significantly contributes to local immunity and initial viral control [24]. Meanwhile, IgM, as the first immunoglobulin secreted during the primary response, has demonstrated considerable neutralizing capacity before IgG production [25]. These findings underscore the importance of evaluating the dynamic behavior of these immunoglobulins in future studies, particularly in the context of emerging variants and vaccination strategies. Incorporating these aspects could provide a more comprehensive understanding of the immune responses induced by vaccination and natural infection in diverse populations.
The findings of this study have significant clinical and epidemiological implications. Clinically, the decrease in neutralizing antibody levels, particularly Immunoglobulin M, suggests that protection acquired through vaccination or infection may be transient, highlighting the need for booster strategies to maintain effective immunity, especially in the face of emerging variants of SARS-CoV-2. Furthermore, the variability in antibody responses among individuals with different infection histories suggests that treatment and prophylaxis approaches may require personalization, considering each patient's clinical history and immune status.
From an epidemiological perspective, the observation of a reduction in neutralizing antibody titers over time, combined with the circulation of emerging variants with higher immune escape potential, emphasizes the importance of continuous surveillance of variants and the assessment of vaccine efficacy in specific populations. This could imply the need to adjust vaccination policies to include more frequent boosters, particularly in vulnerable populations and those with risk factors for severe infection. Ongoing monitoring of variants and their immune evasion capabilities will be crucial for guiding future COVID-19 control and prevention strategies.
A strength of this study was the use of the ACE2-RBD neutralization test, developed by DIA.PRO, without the need for a biosafety level 3 laboratory. Nevertheless, the test demonstrated high sensitivity and specificity for measuring biological activity and antibody levels. A limitation of the study was the lack of use of variant strains, which prevented the comparison of the decrease in neutralizing response according to the studied variant. Additionally, the sample size and the absence of variant-specific analysis could affect the generalization of the results and their interpretation in the context of emerging variants.
Conclusions
In conclusion, this study showed that neutralizing antibody titers change over time; however, they remained detectable and neutralizing in most participants, which could corroborate the efficacy of immunization with BNT162b2 mRNA. Further research on neutralizing antibodies (NAb) is needed, as they may be used as a therapeutic measure with plasma from convalescent donors. Additionally, the constant mutations of SARS-CoV-2 justify further studies to evaluate NAb activity and the need to continue booster immunizations to prevent the spread of COVID-19. For future research, it is suggested to assess variant-specific neutralization and explore antibody dynamics in vulnerable populations.
References
1. Ministerio de salud y protección social. Anexo Técnico No 1 (Elaborado con base en el Anexo No.1 “Manual para la elaboración de textos normativos-proyectos de decreto y resolución”, adoptado mediante Decreto 1081 de 2015, modificado por Decreto 1609 del mismo año) [Internet]; 2024 [cited 2024 Dec 24]. Available from: Available from: https://www.minsalud.gov.co/Anexos_Normatividad_Nuevo/Memoria-justificativa-281420240919120016734.pdf
2. Cheedarla N, Hanna LE. Functional and Protective Role of Neutralizing Antibodies (NAbs) Against Viral Infections. In: Buddolla V, editor. Recent Developments in Applied Microbiology and Biochemistry [Internet]. Cambridge: Academic Press; 2019. p. 83-93. doi: https://doi.org/10.1016/B978-0-12-816328-3.00007-6
3. Beltrán-Pavez C, Riquelme-Barrios S, Oyarzún-Arrau A, Gaete-Argel A, González-Stegmaier R, Cereceda-Solis K, et al. Insights into neutralizing antibody responses in individuals exposed to SARS-CoV-2 in Chile. Sci Adv [Internet]. 2021;7(7):1-13. doi: https://doi.org/10.1126/sciadv.abe6855
4. Li X, Liang C, Xiao X. SARS-CoV-2 Neutralizing Antibody Levels Post COVID-19 Vaccination Based on ELISA Method-A Small Real-World Sample Exploration. Vaccines [Internet]. 2021;9(10):1-10. doi: https://doi.org/10.3390/VACCINES9101139
5. Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med [Internet]. 2021;9(9):999-1009. doi: https://doi.org/10.1016/S2213-2600(21)00220-4
6. Carrillo J, Izquierdo-Useros N, Ávila-Nieto C, Pradenas E, Clotet B, Blanco J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem Biophys Res Commun [Internet]. 2021;538:187-91. doi: https://doi.org/10.1016/J.BBRC.2020.10.108
7. Pang NYL, Pang ASR, Chow VT, Wang DY. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Mil Med Res [Internet]. 2021;8(1):1-17. doi: https://doi.org/10.1186/S40779-021-00342-3
8. Legros V, Denolly S, Vogrig M, Boson B, Siret E, Rigaill J, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol [Internet]. 2021;18(2):318-27. doi: https://doi.org/10.1038/s41423-020-00588-2
9. Edara VV, Hudson WH, Xie X, Ahmed R, Suthar MS. Neutralizing Antibodies Against SARS-CoV-2 Variants After Infection and Vaccination. JAMA [Internet]. 2021;325(18):1896-8. doi: https://doi.org/10.1001/JAMA.2021.4388
10. Shrestha LB, Tedla N, Bull RA. Broadly-Neutralizing Antibodies Against Emerging SARS-CoV-2 Variants. Front Immunol [Internet]. 2021;12:752003. doi: https://doi.org/10.3389/FIMMU.2021.752003
11. Sharun K, Tiwari R, Dhama K, Emran TB, Rabaan AA, Mutair AA. Emerging SARS-CoV-2 variants: impact on vaccine efficacy and neutralizing antibodies. Hum Vaccin Immunother [Internet]. 2021;17(10):3491-4. doi: https://doi.org/10.1080/21645515.2021.1923350
12. Furukawa K, Tjan LH, Kurahashi Y, Sutandhio S, Nishimura M, Arii J, et al. Assessment of Neutralizing Antibody Response Against SARS-CoV-2 Variants After 2 to 3 Doses of the BNT162b2 mRNA COVID-19 Vaccine. JAMA Netw Open [Internet]. 2022;5(5):e2210780. doi: https://doi.org/10.1001/JAMANETWORKOPEN.2022.10780
13. Centers for Disease Control and Prevention [Internet]. Atlanta: Centers for Disease Control and Prevention; c2024. SARS-CoV-2 Variant Classifications and Definitions; 2022 Apr 26 [cited 2022 Aug 22]; [about 12 screens]. Available from: Available from: https://web.archive.org/web/20220822222151/https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html
14. Harris PA, Taylor R, Minor BL, Elliot V, Fernandez M, O’ Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform [Internet]. 2019;95:103208. doi: https://doi.org/10.1016/j.jbi.2019.103208
15. Colmenares-Mejía CC, Quintero-Lesmes DC, Salazar Acosta I, Suárez DP, Meneses L, Sopó Rincón OL, et al. Seroprevalence of SARS-CoV-2 Infection among Vaccinated Health Workers and Hospital Staff. Arch Clin Biomed Res [Internet]. 2022;6(2):290-5. doi: https://doi.org/10.26502/acbr.50170244
16. Serrano NC, Quintero-Lesmes DC, Colmenares-Mejía CC, Salazar Acosta I, Suárez DP, Meneses L, et al. Infección por SARS-CoV-2 y respuesta de anticuerpos a la vacuna BNT162b2 en trabajadores de la salud de primera línea de atención para covid-19. Medicina (B Aires) [Internet]. 2022;44(1):31-9. doi: https://doi.org/10.56050/01205498.1659
17. AESKU.GROUP. WE TAKE CARE OF YOUR HEALTH [Internet]. Wendelsheim: Aesku Group; c2024 [cited 2022 Aug 22]; [about 2 screens]. Available from: Available from: https://www.aesku.com/
18. DIA.PRO Diagnostic Bioprobes srl. ACE2-RBD Neutralization Assay - ELISA [Internet]. Sesto San Giovanni: Dia.Pro; c2024 [cited 2022 Aug 22]; [about 2 screens]. Available from: Available from: https://www.diapro.it/products/ace2-rbd-neutralization-assay-elisa/
19. Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature [Internet]. 2021;591(7851):639-44. doi: https://doi.org/10.1038/s41586-021-03207-w
20. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med [Internet]. 2021;27(7):1205-11. doi: https://doi.org/10.1038/s41591-021-01377-8
21. Rogliani P, Chetta A, Cazzola M, Calzetta L. SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines [Internet]. 2021;9(3):1-18. doi: https://doi.org/10.3390/VACCINES9030227
22. Tea F, Stella AO, Aggarwal A, Darley DR, Pilli D, Vitale D, et al. SARS-CoV-2 neutralizing antibodies: Longevity, breadth, and evasion by emerging viral variants. PLoS Med [Internet]. 2021;18(7):e1003656. doi: https://doi.org/10.1371/JOURNAL.PMED.1003656
23. Tang MS, Case JB, Franks CE, Chen RE, Anderson NW, Henderson JP, et al. Association between SARS-CoV-2 Neutralizing Antibodies and Commercial Serological Assays. Clin Chem [Internet]. 2020;66(12):1538-47. doi: https://doi.org/10.1093/CLINCHEM/HVAA211
24. Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med [Internet]. 2021;13(577): eabd2223. doi: https://doi.org/10.1126/scitranslmed.abd2223
25. Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection in humans. Nat Microbiol [Internet]. 2020;5(12):1598-607. doi: https://doi.org/10.1038/s41564-020-00813-8
Supplementary material
Methods
Principle of the reactivity test
The inhibition of the binding between ACE2 and RBD is determined employing an ELISA carried out on plasma/sera whose antibodies-neutralizing action wants to be measured. Microplates are coated with SARS-CoV-2 specific recombinant glycosylated RBD. A color will be generated if no antibodies have bound to RBD while a strong inhibition on the color development will be observed in case of antibodies to RBD have blocked the binding of the biotin-labeled ACE2 to it. The presence of such antigen in the solid phase is finally determined by the addition of SAV-HRP, which will bind to ACE2 if no neutralizing antibodies are present or not in case antibodies have blocked the coated RBD.
Results
Agreement between antibody levels and neutralizing activity is shown in Figure S1.
Figure S1. Correlation between first sampling and neutralizing activity.
Table S1. Correlation between antibody levels and reactivity according to history of COVID-19 infection.
| Ab | Previous COVID infection | No previous COVID infection | ||
|---|---|---|---|---|
| rho | p | rho | P | |
| IgA | 0.41 | 0.416 | 0.67 | <0.001 |
| IgM | 0.40 | 0.060 | 0.40 | 0.001 |
| IgG | -0.07 | 0.730 | 0.60 | <0.001 |