Hearing Loss Rehabilitation and Its Contribution to Cognitive-Linguistic Performance in Healthy Older Adults: A Systematic Review
Rehabilitación de la pérdida auditiva y su contribución al desempeño cognitivo-lingüístico en adultos mayores sanos: una revisión sistemática
Francisca Mansilla–Jara, Teresa Julio-Ramos, Álvaro Godoy–Diaz, Daniel Solomons, Igor Cigarroa, David Toloza–Ramirez
Abstract
Introduction. Hearing loss (HL) rehabilitation involves using different hearing technologies, such as hearing aids or cochlear implants. The efficacy of HL rehabilitation strategies and their cognitive benefits has been reported for clinical populations, such as those with mild cognitive impairment or dementia. However, up to date, there is no clarity about the cognitive-linguistic implications of HL rehabilitation for healthy older adults. Therefore, increasing knowledge about its benefits in healthy older people is critical to generating early intervention strategies that could delay the progression to pathological stages.
Aim. To profile cognitive-linguistic performance after HL rehabilitation in healthy older adults.
Methodology. Systematic literature review following the PRISMA guidelines. We included randomized and non-randomized clinical trials from Medline by PubMed, Scopus, and Web of Science databases (January 2000 to May 2024).
Results. We identified 410 titles, from which five papers were qualitatively analyzed. Results suggest that after HL rehabilitation, healthy older adults improve their performance in specific abilities such as working memory, delayed recall, attention, semantic and phonological fluency, and language comprehension. We highlight an association between working memory improvement and semantic skills' benefits, especially in subjects with mild to moderate HL.
Conclusion. HL rehabilitation programs should consider cognitive-linguistic stimulation programs in healthy older adults to prevent cognitive dysfunction or neurodegenerative conditions. We only analyzed a few studies; thus, we suggest interpreting the information carefully. Indeed, promoting more follow-up studies to clarify the benefits of using hearing devices and their cognitive-linguistic implications in healthy people is still necessary.
Keywords
Hearing loss; rehabilitation; aging; cognition; language.
Resumen
Introducción. La rehabilitación de la pérdida auditiva (PA) implica el uso de diferentes tecnologías como audífonos o implantes cocleares, siendo descritos sus beneficios cognitivos para poblaciones clínicas como el deterioro cognitivo leve o demencia. Sin embargo, no hay claridad a la fecha sobre las implicancias cognitivo-lingüísticas de la rehabilitación auditiva en personas mayores sanas con PA. Por tanto, incrementar el conocimiento sobre sus beneficios en personas mayores sanas es fundamental para generar lineamientos de intervención tempranos que puedan retrasar la progresión de esta población a estadios patológicos.
Objetivo. Perfilar el rendimiento cognitivo-lingüístico tras la rehabilitación auditiva en adultos mayores sanos con PA.
Metodología. Se realizó una revisión sistemática siguiendo los lineamientos PRISMA. Se incluyeron ensayos clínicos aleatorizados y no aleatorizados desde Medline (PubMed), Scopus y Web of Science (enero 2000 a mayo 2024).
Resultados. Cinco estudios fueron analizados. Los resultados sugieren que, tras la rehabilitación de la PA, los adultos mayores mejoran su rendimiento en capacidades memoria de trabajo, recuerdo retardado, atención, fluidez semántica-fonológica, y lenguaje comprensivo. La mejora en memoria de trabajo favorece las habilidades semánticas, especialmente en PA leve a moderada.
Conclusión. La rehabilitación de la PA debería considerar programas de estimulación cognitivo-lingüística en adultos mayores sanos para prevenir declives cognitivos o cuadros neurodegenerativos. Dado el número de estudios incluidos, nuestros hallazgos deben ser interpretados con cautela. Finalmente, sigue siendo necesario promover más estudios de seguimiento para dilucidar los beneficios del uso de dispositivos auditivos y sus implicancias cognitivas-lingüísticas en personas mayores sanas.
Palabras clave
Pérdida Auditiva; rehabilitación; envejecimiento; cognición; lenguaje.
Introduction
Hearing loss (HL) has emerged as a topic of interest in aging due to its increasing prevalence, as well as being considered a risk factor for developing dementia [1,2]. It is estimated that 1.57 billion people worldwide suffer from HL, with a prevalence of 62.1% in people over 50 [3]. The severity of HL in older people ranges from moderate to severe in most cases [4], affecting communicative skills, language, cognition, mental health, and quality of life [5-8].
Loughrey et al. (2017) [9] report a significant association between HL and the development of dementia and cognitive impairment. The literature suggests that HL may accelerate cognitive decline in healthy older adults by 30-40% [6]. Several studies highlight the negative impact of HL on neuropsychological tests, primarily affecting performance in verbal and non-verbal skills [10-13]. Also, cognitive-linguistic decline in older people with HL relates to increased social isolation, leading to a higher risk of cognitive impairment and dementia [14,15].
HL treatment has been widely studied in the pediatric and adult populations. The evidence in children and adolescents often emphasizes the importance of auditory rehabilitation during sensitive stages, such as brain development, for acquiring cognitive-linguistic skills [16-18]. In contrast, Birman and Hassarati (2023) [19] highlight the importance of hearing technologies, such as hearing aids and cochlear implants, for auditory rehabilitation in older adults due to their vital role in maintaining cognitive and communicative abilities and quality of life.
There is evidence that HL rehabilitation in healthy older people increases cognitive effort and anatomical-functional changes, improving quality of life and cognitive-linguistic skills [20]. Thus, early rehabilitation in healthy aging would be crucial to mitigate cognitive-linguistic declines and even delay the progression to more severe conditions (e.g., dementia) [21]. However, a systematic review [22] focused on the use of hearing aids and their effect on cognitive function showed inconclusive results regarding the utility of hearing aids on neuropsychological performance (cognitive-linguistics).
The knowledge of cognitive-linguistic profiles in older adults has provided guidelines for improving the distinction between normal and pathological aging [23]. Previous systematic reviews [24-26] showed that auditory rehabilitation improves memory or executive function, but these studies were limited to pathological aging (e.g., cognitive impairment or dementia) and did not consider cognitive and linguistic benefits for healthy adults.
Based on the evidence described above, the following research question arises: What are the effects of HL rehabilitation in cognitive and linguistic performance in healthy older adults? Therefore, this systematic review aims to profile cognitive-linguistic performance after HL rehabilitation in healthy older adults.
Methodology
This systematic review followed PRISMA 2020 reporting guidelines [27] and was previously registered in the PROSPERO repository (CRD42024517607; registration date February 25, 2024).
Sources and search strategy
The identification of bibliographic references included Medline by PubMed, Scopus, and Web of Science databases, focusing on articles published from January 2000 to May 2024. The search syntax was as follows: [hearing impairment OR hearing loss OR deafness OR hypoacusis] AND [hearing aids OR aid OR aids OR ear molds OR cochlear implant OR rehabilitation auditory] AND [cognition OR memory OR working memory OR memories OR short-term memory OR immediate memory OR immediate recall OR processing speed OR executive function OR verbal fluency]. Moreover, we adapted all the terms to improve the search in each database.
Study selection and eligibility criteria
The inclusion criteria were as follows: a) healthy people over 60 years; b) healthy older adults with moderate to profound HL, considering only older adults with HL related to the aging process, and healthy for this research purpose consider people without neurological conditions (e.g., stroke, cognitive impairment, among others); c) people using hearing aids, cochlear implants, or any auditory technology to facilitate the functional cognitive-communicative process; d) randomized and non-randomized clinical trials published in English; and e) studies reporting neuropsychological assessment for cognition and/or language skills. On the other hand, the exclusion criteria were: a) editorial documents, systematic reviews (with or without meta-analyses), protocols, or theses, b) people with subjective cognitive impairment, and c) healthy older adults with a clinical history of depression, psychosis, and/or schizophrenia.
Data extraction
The research team imported all the studies to Rayyan software [28] and eliminated the duplicates. Considering the inclusion and exclusion criteria, two reviewers (Francisca Mansilla-Jara and Teresa Julio-Ramos) filtered the articles independently. It is essential to highlight that the research team accessed the full text for clarification when the title and abstract were insufficient to decide its inclusion or exclusion. In addition, a third researcher (David Toloza-Ramirez) reached a consensus on selecting these studies.
Risk of Bias Assessment Tool
The risk of bias was assessed using the Cochrane Collaboration tool [29], and we created the traffic light plot and a summary plot using “the robvis tool” [30]. Six domains were considered for evaluation: D1 related to selection bias, D2 related to performance bias, D3 related to detection bias, D4 for attrition bias, D5 related to reporting bias, and the overall risk of bias. The risk of bias evaluation criteria for each domain considered a judgment of high, low, or unclear (some concerns). Based on this, Francisca Mansilla-Jara and Teresa Julio-Ramos assessed the articles included independently, and in case of disagreement, David Toloza-Ramirez contributed to the consensus.
Data synthesis strategy
A narrative synthesis of the findings of the included studies is summarized in Table 1, considering relevant general characteristics of the studies, including source, sample size, number of withdrawn, sex, mean age, and HL severity. In addition, we provided information about intervention characteristics and primary outcomes considering the following aspects: auditory technology used, auditory implementation characteristics, and the main findings regarding cognitive-linguistic performance after rehabilitation in healthy older adults with HL.
Table 1. Summary of studies included regarding older adults with HL.
| Source | Sample (n) | Withdrawn (n) | Sex (n) | Mean age (SD) | HL severity | Auditory technology | Auditory implementation | Measure | Summary of cognitive-linguistic findings | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | Mod | Sev | Pro | HA | CI | Uni | Bil | ||||||
| Henshaw et al. (2022) [31] | 57 | 0 | 30 | 27 | 66.2 (6.3) | ✓ | - | - | ✓ | - | - | ✓ | ||
| HHIE | ||||||||||||||
| Völter et al. (2021) [32] | 71 | 12 | 47 | 24 | 66.3 (9.2) | - | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Pre 682.25 ( 190.24; Post 609.29 ( 230.80 (p = .0007) | ||||||||||||||
| VF | Pre 804.29 ( 77.93; Post 750.77 ( 114.49 (p = .00006) | |||||||||||||
| Wick et al. (2020) [33] | 70 | 0 | 19 | 51 | 74 (-) | ✓ | ✓ | ✓ | - | ✓ | ✓ | - | ||
| QoL | Pre 0.850 (0.803 to 0.897); Post 0.949 (0.924 to 0.974); Difference 0.099 (0.049 to 0.149) | |||||||||||||
| Souza et al. (2019) [34] | 49 | 9 | 19 | 21 | 72 (-) | ✓ | - | - | ✓ | - | ✓ | ✓ | ||
| 0.10 (0.08, 0.12), 0.011 (p = <.001) | ||||||||||||||
| Magalhães and Iório (2011) [35] | 50 | 0 | 23 | 27 | - | - | ✓ | - | ✓ | - | - | ✓ | ||
| MSME | Pre 32.9 ( 5.9; Post 8.8 ( 5.1 (p = .0001) | |||||||||||||
Note. ✓: Specified. -: Not reported/Not specified. SD: Standard Deviation. HL: Hearing loss. Mod: Moderate. Sev: Severe. Pro: Profound. HA: Hearing Aids. CI: Cochlear Implant. Uni: Unilateral. Bil: Bilateral. PTA: Pure-tone audiometry. WM: Working memory. VLM: Visual letter monitoring task. MCRM: Modified coordinate response measure. TEA: Test of Everyday Attention. DS: Digit Span. WAIS-III: Wechsler Adult Intelligence Scale - Third Edition. TAIL: Test of attention in listening. RT: Reaction time. SICSPAN: Size comparison span. OSPAN: Operation span. GHABP: Glasgow Hearing Aid Benefit Profile. HHIE: Hearing Handicap Inventory for the Elderly. BEEST: British English Semantic Sentence Test. GDS: Geriatric Depression Scale. MWT-B: Multiple Word Sentence Test - Part B. M3T: M3 Test. RT: Recall Test. DRT: Delayed Recall Test. 0-Back: 0 Back Test. 2-Back: 2 Back Test. cFlanker: compatible Flanker test. iFlanker: incompatible Flanker test. OSPAN: Operation Span. TMT A: Trail Making Test, part A. TMT B: Trail Making Test, part B. VF: Verbal fluency. SP: Speech perception. HUI3: Health Utilities Index Mark 3. SSQ: Speech, Spatial, and Qualities of Hearing Scale. DUQ: Device Use Questionnaire. MoCA: Montreal Cognitive Assessment. QoL: Quality of life. RST: Reading span test. PHAST-R: Practical Hearing Aid Skills Test-Revised. SR: Speech recognition. IPRF: Speech recognition percent index. MSME: Mental State Mini Exam.
Results
Literature search
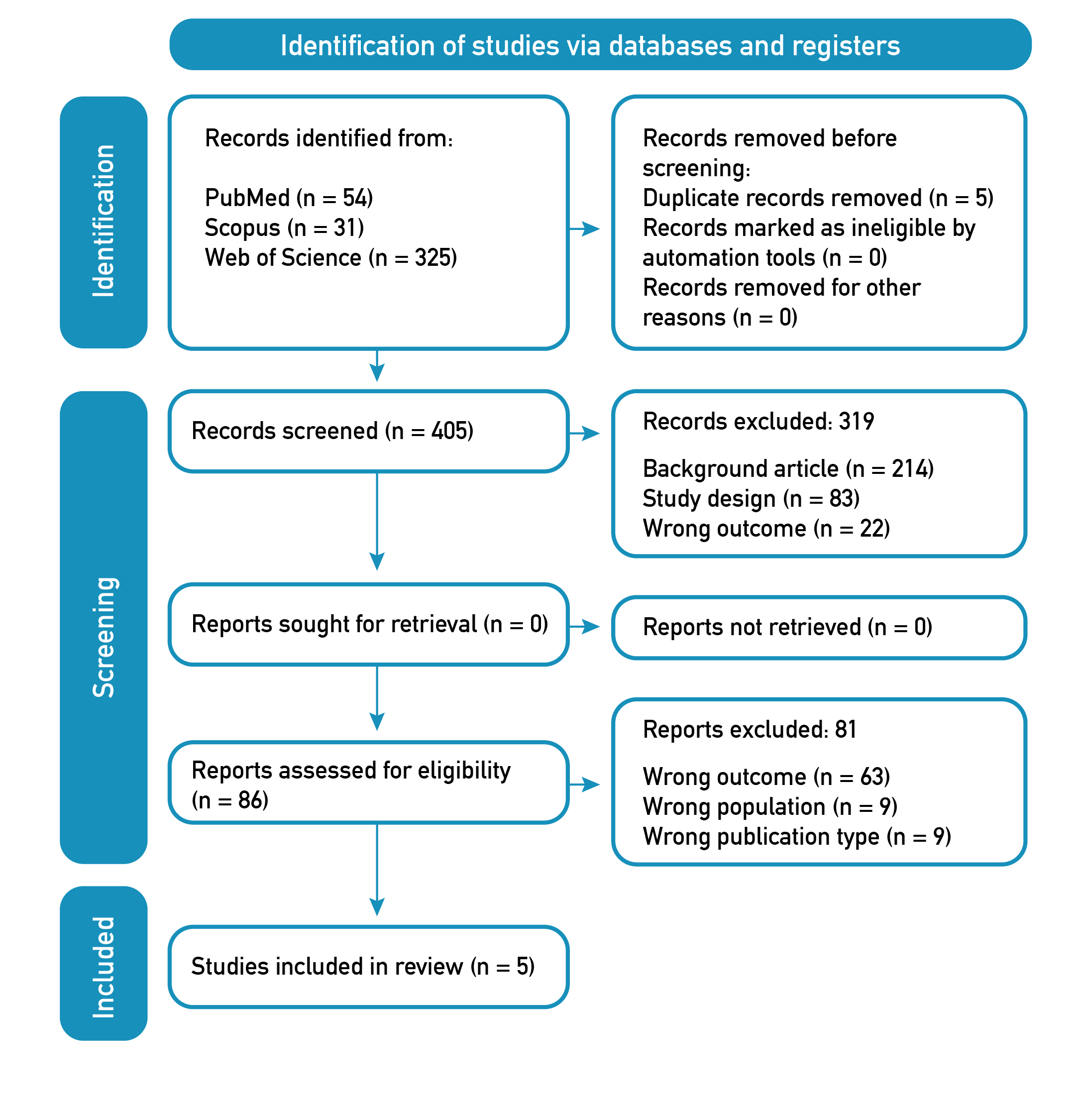
The PRISMA flowchart [27] illustrates the article selection process (Figure 1). We identified titles and abstracts of 410 articles, considering five duplicates. After applying the inclusion/exclusion criteria, 319 articles were excluded: 214 based on background article, 83 based on study design, and 22 based on wrong outcome. Of the 86 full-text articles selected for eligibility, 63 were excluded based on wrong outcome, nine on wrong population, and nine on wrong publication type. Finally, five articles were included for qualitative analysis in this systematic review.
Figure 1. Flow Diagram PRISMA search and selection articles.
General characteristics of the studies
Table 1 summarizes the general characteristics of the five included studies [31-35]. The total sample consisted of 297 participants, of whom 21 withdrew from the study (7%). Regarding age and sex variables, the mean age was 69.6 years (SD: 7.75), and 150 volunteers were men (52%).
HL rehabilitation characteristics
Concerning HL severity, Table 1 shows heterogeneity among the five studies, including the most reported severity in healthy older adults with HL ranging from moderate to severe. The auditory technology most used for rehabilitation was hearing aids (reported in 4/5 studies), and cochlear implants were implemented only in 2/5 of the studies analyzed. Finally, it is essential to highlight that concerning the auditory implementation for hearing rehabilitation, the five articles included did not show differences in uni or bilateral implementation choices (50% unilateral and 50% bilateral).
Cognitive-linguistic performance was analyzed in all the studies included as well. Our qualitative analysis suggests that the cognitive contribution of HL rehabilitation mainly focuses on three abilities: working memory, delayed recall, and attention. Language performance showed benefits in semantic and phonological fluency, speech perception, and language comprehension skills. As a complement to Table 1, we present a descriptive summary of each study below:
Study 1 [31]
Cognition in healthy older adults with HL showed benefits in the working memory domain after auditory rehabilitation. However, no significant benefits in this cognitive domain were reported after six months of auditory rehabilitation. Indeed, results suggest that the slight improvement in working memory does not contribute to other cognitive domains, such as attention. Moreover, results highlight a significant increase in attention capacity in healthy older adults after rehabilitation compared to baseline. On the other hand, language performance did not show changes in the HL group. Indeed, semantic skills were maintained after auditory training in healthy older adults with HL, and only a slight improvement in speech perception was found.
Study 2 [32]
Results suggest healthy older adults with HL improved cognitive-linguistic performance after 12 months of implementing auditory technology, but the effects are limited to unilateral CI implementation. On the one hand, significant effects were observed after auditory rehabilitation in cognitive domains such as attention, delayed recall, working memory, and inhibition. However, processing speed and executive function did not show benefits after auditory implementation. On the other hand, language performance considered improvements in auditory speech comprehension, speech perception, and verbal fluency skills (semantic and phonological).
Study 3 [33]
After auditory implementation, cognitive-linguistic performance in healthy older adults with HL showed no significant benefits. Cognitive effects were observed in executive function, memory, attention, delayed recall, and visuoconstructive skills. Moreover, naming skills and semantic and phonological fluency improved after the implementation; however, those effects were insignificant. Based on these findings, it is essential to highlight that all participants were assessed after six months of auditory rehabilitation. This could explain no long-term duration of cognitive-linguistic effects.
Study 4 [34]
Results suggest healthy older adults with HL improved working memory after auditory rehabilitation. Moreover, the authors highlight that a better working memory capacity does not depend on age in this group. On the other hand, working memory benefits correlated with better speech recognition and intelligibility, which could contribute to language performance. Indeed, better speech recognition can promote language comprehension and functional communication in older people.
Study 5 [35]
Results highlight better cognitive-linguistic performance in the HL group after rehabilitation. The main improvements considered memory capacity, memory recall, attention, and language (comprehension, repetition, and following instructions skills). Moreover, the study suggests that cognitive-linguistic domains' benefits promote more social participation in older people with HL.
Risk of bias assessment
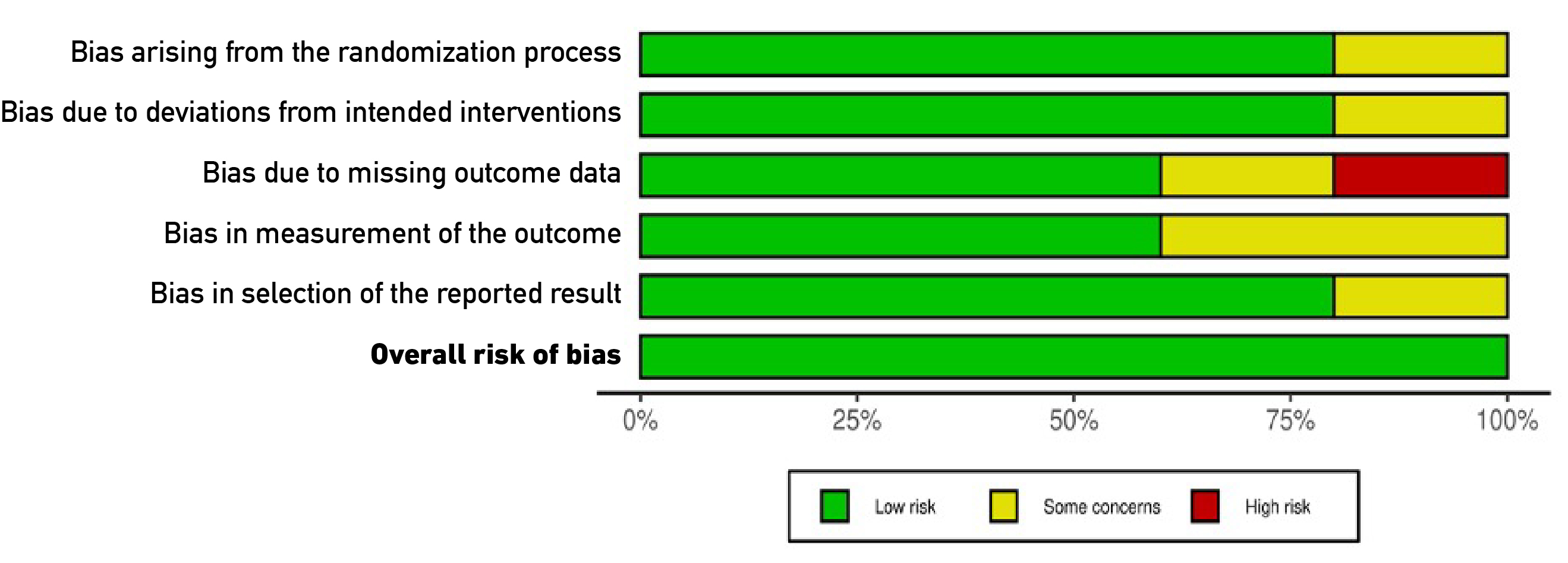
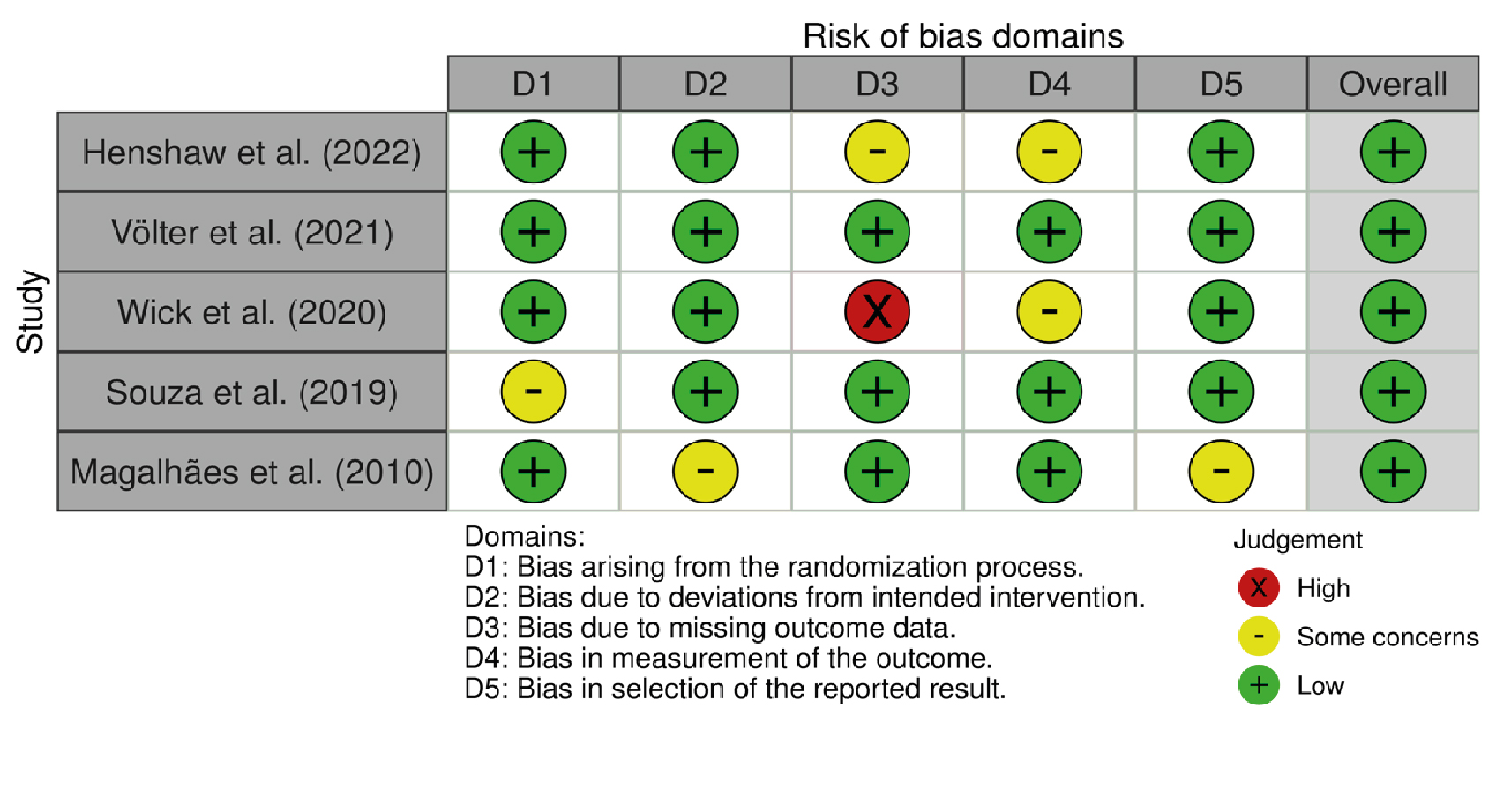
We analyzed the five studies based on six domains from the Cochrane tool [29], and the results are presented in Figures 2 and 3 [30]. A low risk of bias was observed for selection bias (D1), performance bias (D2), and reporting bias (D5) (80%, respectively, for each domain). Regarding domains three and four (detection bias and attrition bias), 60% of studies were classified as low risk of bias. Moreover, it is essential to highlight that 40% of the articles in the detection bias item (D3) were classified as high and unclear risks (20% each). Finally, in 5/5 studies (100%), a "low risk" classification for overall risk of bias was observed. The summary plot of the risk of bias is presented in Figure 2, in addition to a traffic light plot, which shows the analysis of each study performed (Figure 3).
Figure 2. Summary plot of the risk of bias.
Figure 3. Traffic light plot of the risk of bias.
Discussion
We profiled cognitive-linguistic performance after HL rehabilitation in healthy older adults. Results suggest that cognitive-linguistic performance improves in specific domains, but only semantic skills are maintained after auditory rehabilitation.
Working memory is crucial for retaining information during linguistic tasks and comprehending, producing, and storing new information. Likewise, it has been reported that language and working memory tasks activate standard neural systems, indicating that working memory responds to cognitive demand, mainly influencing phonological processing [36]. Indeed, the literature suggests that working memory is a cognitive domain that improves after HL rehabilitation because it can potentially reduce cognitive burden, facilitating auditory processing and the functioning of other cognitive subdomains [37]. Thus, this is an essential concern in typical aging, where working memory tends to reduce capacity, which could progress to pathological stages such as mild cognitive impairment [38].
The follow-up study by Fernándes et al. [39] reported improvement in memory capacity and attention after HL rehabilitation, with positive effects on cognitive performance as a result of hearing aid devices. The authors emphasize that this potential effect is limited to healthy older adults with mild to moderate HL. These findings align with previous studies [40-43] that showed the potential short-term impacts of auditory rehabilitation in older people, especially in memory and attention abilities, which is also suggested in our revision. Nevertheless, differences emerge when comparing our findings with broader reviews, such as Sanders et al. [22]. While Sanders et al. identified a more comprehensive range of impacts of auditory rehabilitation on cognitive abilities in diverse populations, our review integrates language as an essential cognitive function. Indeed, the search strategy used by the authors considers only cognitive domains such as memory, attention, and executive function; however, they do not include language as a critical cognitive term. Therefore, considering our search strategy, this could explain the lower number of studies included for qualitative analysis. Despite this, our review provides a general understanding of cognitive and linguistic domains after HL rehabilitation. Moreover, our review highlights the need for standardized cognitive assessment protocols and long-term randomized methodology-based follow-ups, echoed by Sanders et al. and subsequent literature. These differences allow us to emphasize that our findings complement previous studies, focusing on domains that require further integration, such as language, which has been considered in cognitive neurosciences as a cognitive function.
Our review did not find significant changes in executive function or processing speed post-auditory rehabilitation. However, a recent investigation by Yang et al. [24] revealed that healthy older people who use hearing aid devices can enhance cognitive performance, specifically executive function, but in people with Alzheimer's Disease or dementia, no substantial changes in cognitive performance were reported despite the use of hearing aids. Likewise, Maharani et al. [44] found that hearing aids are associated with a slower mental decline in older adults, highlighting its potential in executive function and speed processing. Furthermore, another previous study by Dawes et al. [45] reported better performance in executive tasks in healthy older adults who constantly used their hearing aids than those who did not. These results align with a previous longitudinal study, highlighting that HL based on hearing aids could improve cognitive performance in older people for three years [46].
Language performance, especially semantic skills, also improved after HL rehabilitation, in association with better working memory performance. Our revision suggests this association occurs in people with mild to moderate HL; thus, the severity of HL could influence semantic benefits after auditory rehabilitation [47]. In addition, other studies [48,49] have supported our findings concerning semantic and phonological fluency, demonstrating that auditory rehabilitation could improve verbal fluency abilities in healthy older adults.
Comprehensive skills were also reported as potential benefits of HL rehabilitation. Literature suggests that auditory rehabilitation benefits auditory perception, facilitating better integration of acoustic information and interpreting the information in several linguistic contexts [50,51]. Likewise, it has been postulated that auditory rehabilitation in older people also contributes to more effective communication and a better quality of life [52,53]. Furthermore, our results revealed differences based on the type of auditory adaptation (unilateral or bilateral), with more significant cognitive-linguistic performance improvements observed in individuals with bilateral adaptation. So far, this observation is consistent with Chen's findings in 2023 [54], suggesting that bilateral auditory rehabilitation presents a significant therapeutic option for individuals with age-related HL and mild cognitive impairment. Chen's study underscores the importance of implementing protocols that effectively manage age-related hearing loss to mitigate cognitive decline.
Our qualitative review also found enhancement in social participation after the cognitive-linguistic benefits of HL rehabilitation. Age-related HL is associated with a higher risk of social isolation in older adults, which may imply a higher risk of dementia or cognitive decline [55]. Similarly, a previous study indicates that in people with HL, verbal communication failures could imply social integration difficulties [56]. Dawes et al. [45] found that HL intervention, such as hearing aids, may improve cognitive performance, but this is not explained by reduced social isolation.
Limitations
This systematic review has some limitations. We did not perform a meta-analysis of the HL rehabilitation programs. The rehabilitation schedule characterized in our review only considered the implementation of hearing devices, excluding articles based on cognitive-linguistic conventional stimulation (e.g., cognitive or language therapy). On the other hand, we established a limit of years for the article's inclusion, which could contribute to a bias in the research process. Finally, we only included articles in English, but we are conscious that there are several investigations in other languages (e.g., Spanish, German, and others).
Future Directions and Contribution
These findings have clinical implications, such as defining the most appropriate approach to hearing intervention in people with age-related HL [57,58]. This approach could consider cognitive-linguistic intervention in addition to hearing aids or cochlear implants. Besides this review, we suggest including cognitive assessment in older adults with HL to diagnose and monitor possible cognitive declines [59].
Conclusions
HL rehabilitation could improve cognitive-linguistic performance; however, combining hearing devices with neuropsychological stimulation programs is still necessary. The evidence is limited to the effects of HL rehabilitation and its contribution to anatomical-functional effects on the brain. Therefore, promoting follow-up studies could prevent the progression from healthy aging to pathological stages, which has been reported as critical for functional impact on activities of daily living, quality of life, and neuropsychological performance.
References
1. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet [Internet] 2020;396(10248):413-46. doi: https://doi.org/10.1016/s0140-6736(20)30367-6
2. Castiglione A, Benatti A, Velardita C, Favaro D, Padoan E, Severi D, et al. Aging, cognitive decline and hearing loss: Effects of auditory rehabilitation and training with hearing aids and cochlear implants on cognitive function and depression among older adults. Audiol Neurotol [Internet]. 2016;21(Suppl 1):21-8. doi: https://doi.org/10.1159/000448350
3. GBD 2019 Hearing Loss Collaborators. Hearing loss prevalence and years lived with disability, 1990-2019: findings from the Global Burden of Disease Study 2019. Lancet [Internet]. 2021;397(10278):996-1009. doi: https://doi.org/10.1016/s0140-6736(21)00516-x
4. Vogelzang M, Thiel CM, Rosemann S, Rieger JW, Ruigendijk E. Effects of age-related hearing loss and hearing aid experience on sentence processing. Sci Rep [Internet]. 2021;11(1):5994. doi: https://doi.org/10.1038/s41598-021-85349-5
5. Cosh S, Helmer C, Delcourt C, Robins TG, Tully PJ. Depression in elderly patients with hearing loss: current perspectives. Clin Interv Aging [Internet]. 2019;14:1471-80. doi: https://doi.org/10.2147/cia.s195824
6. Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med [Internet]. 2013;173(4):293-9. doi: https://doi.org/10.1001/jamainternmed.2013.1868
7. Mormer E, Bubb KJ, Alrawashdeh M, Cipkala-Gaffin JA. Hearing loss and communication among hospitalized older adults: Prevalence and recognition. J Gerontol Nurs [Internet]. 2020;46(6):34-42. doi: https://doi.org/10.3928/00989134-20200316-03
8. Powell DS, Oh ES, Lin FR, Deal JA. Hearing impairment and cognition in an aging world. J Assoc Res Otolaryngol [Internet]. 2021;22(4):387-403. doi: https://doi.org/10.1007/s10162-021-00799-y
9. Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg [Internet]. 2018;144(2):115-26. doi: https://doi.org/10.1001/jamaoto.2017.2513
10. Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore longitudinal study of aging. Neuropsychology [Internet]. 2011;25(6):763-70. doi: https://doi.org/10.1037/a0024238
11. Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci [Internet]. 2011;66A(10):1131-6. doi: https://doi.org/10.1093/gerona/glr115
12. Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. Sensory and cognitive association in older persons: Findings from an older Australian population. Gerontology [Internet]. 2006;52(6):386-94. doi: https://doi.org/10.1159/000095129
13. Valentijn SAM, Boxtel MPJV, Van Hooren SAH, Bosma H, Beckers HJM, Ponds RWHM, et al. Change in sensory functioning predicts change in cognitive functioning: Results from a 6‐year follow‐up in the Maastricht aging study. J Am Geriatr Soc [Internet]. 2005;53(3):374-80. doi: https://doi.org/10.1111/j.1532-5415.2005.53152.x
14. Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol [Internet]. 2004;3(6):343-53. doi: https://doi.org/10.1016/s1474-4422(04)00767-7
15. Hughes TF, Andel R, Small BJ, Borenstein AR, Mortimer JA. The association between social resources and cognitive change in older adults: Evidence from the Charlotte County healthy aging study. J Gerontol B Psychol Sci Soc Sci [Internet]. 2008;63(4):P241-4. doi: https://doi.org/10.1093/geronb/63.4.p241
16. Sharma SD, Cushing SL, Papsin BC, Gordon KA. Hearing and speech benefits of cochlear implantation in children: A review of the literature. Int J Pediatr Otorhinolaryngol [Internet]. 2020;133:109984. doi: https://doi.org/10.1016/j.ijporl.2020.109984
17. Tye-Murray N, Spehar B, Sommers M, Mauzé E, Barcroft J, Grantham H. Teaching children with hearing loss to recognize speech: Gains made with computer-based auditory and/or speechreading training. Ear Hear [Internet]. 2022;43(1):181-91. doi: https://doi.org/10.1097/aud.0000000000001091
18. Wood JW, Shaffer AD, Kitsko D, Chi DH. Sudden sensorineural hearing loss in children-management and outcomes: A meta‐analysis. Laryngoscope [Internet]. 2021;131(2):425-34. doi: https://doi.org/10.1002/lary.28829
19. Birman CS, Hassarati RT. Cochlear implant adult speech perception outcomes: Seniors have similar good outcomes. Otol Neurotol [Internet]. 2023;44(5):438-46. doi: https://doi.org/10.1097/mao.0000000000003846
20. del Solar JW, Delgado C, Torrente MC, Délano PH. Hipoacusia como factor de riesgo para demencia. Rev Med Chile [Internet]. 2020;148(8):1128-38. doi: https://doi.org/10.4067/s0034-98872020000801128
21. Stadio AD, Ralli M, Roccamatisi D, Scarpa A, della Volpe A, Cassandro C, et al. Hearing loss and dementia: radiologic and biomolecular basis of their shared characteristics. A systematic review. Neurol Sci [Internet]. 2021;42(2):579-88. doi: https://doi.org/10.1007/s10072-020-04948-8
22. Sanders ME, Kant E, Smit AL, Stegeman I. The effect of hearing aids on cognitive function: A systematic review. PLoS ONE [Internet]. 2021;16(1):e0261207. doi: https://doi.org/10.1371/journal.pone.0261207
23. Malpu-Wiederhold C, Farías-Ulloa C, Cigarroa I, Martella D, Foncea-González C, Julio-Ramos T, et al. Perfiles cognitivos-lingüísticos en personas mayores con Deterioro Cognitivo Leve, Demencia Vascular, Demencia con Cuerpos de Lewy y Enfermedad de Parkinson. Rev Ecuat Neurol [Internet]. 2022;31(3):69-85. doi: https://doi.org/10.46997/revecuatneurol31300069
24. Yang Z, Ni J, Teng Y, Su M, Wei M, Li T, et al. Effect of hearing aids on cognitive functions in middle-aged and older adults with hearing loss: A systematic review and meta-analysis. Front Aging Neurosci [Internet]. 2022;14:1017882. doi: https://doi.org/10.3389/fnagi.2022.1017882
25. Mamo SK, Reed NS, Price C, Occhipinti D, Pletnikova A, Lin FR, et al. Hearing loss treatment in older adults with cognitive impairment: A systematic review. J Speech Lang Hear Res [Internet]. 2018;61(10):2589-603. doi: https://doi.org/10.1044/2018_jslhr-h-18-0077
26. Amieva H, Ouvrard C. Does treating hearing loss in older adults improve cognitive outcomes? A review. J Clin Med [Internet]. 2020;9(3):1-12. doi: https://doi.org/10.3390/jcm9030805
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ [Internet]. 2021;372:n71. doi: https://doi.org/10.1136/bmj.n71
28. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev [Internet]. 2016;5:210. doi: https://doi.org/10.1186/s13643-016-0384-4
29. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ [Internet]. 2011;343:d5928. doi: https://doi.org/10.1136/bmj.d5928
30. McGuinness LA, Higgins JPT. Risk‐of‐bias VISualization (robvis): An R package and Shiny web app for visualizing risk‐of‐bias assessments. Res Synth Methods [Internet]. 2021;12(1):55-61. doi: https://doi.org/10.1002/jrsm.1411
31. Henshaw H, Heinrich A, Tittle A, Ferguson M. Cogmed training does not generalize to real-world benefits for adult hearing aid users: Results of a blinded, active-controlled randomized trial. Ear Hear [Internet]. 2022;43(3):741-63. doi: https://doi.org/10.1097/aud.0000000000001096
32. Völter C, Götze L, Haubitz I, Müther J, Dazert S, Thomas JP. Impact of cochlear implantation on neurocognitive subdomains in adult cochlear implant recipients. Audiol Neurotol [Internet]. 2021;26(4):236-45. doi: https://doi.org/10.1159/000510855
33. Wick CC, Kallogjeri D, McJunkin JL, Durakovic N, Holden LK, Herzog JA, et al. Hearing and quality-of-life outcomes after cochlear implantation in adult hearing aid users 65 years or older. JAMA Otolaryngol Head Neck Surg [Internet]. 2020;146(10):925-32. doi: https://doi.org/10.1001/jamaoto.2020.1585
34. Souza P, Arehart K, Schoof T, Anderson M, Strori D, Balmert L. Understanding variability in individual response to hearing aid signal processing in wearable hearing aids. Ear Hear [Internet]. 2019;40(6):1280-92. doi: https://doi.org/10.1097/aud.0000000000000717
35. Magalhães R, Iório MCM. Evaluation of participation restriction and cognitive processes in the elderly before and after the audiologic rehabilitation. J Soc Bras Fonoaudiol [Internet]. 2010;23(1):51-6. doi: https://doi.org/10.1590/s2179-64912011000100012
36. Deldar Z, Gevers-Montoro C, Khatibi A, Ghazi-Saidi L. The interaction between language and working memory: a systematic review of fMRI studies in the past two decades. AIMS Neurosci [Internet]. 2021;8(1):1-32. doi: https://doi.org/10.3934/neuroscience.2021001
37. Pichora-Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BWY, Humes LE, et al. Hearing impairment and cognitive energy: The framework for understanding effortful listening (FUEL). Ear Hear [Internet]. 2016;37(Suppl 1):5S-27S. doi: https://doi.org/10.1097/aud.0000000000000312
38. Roberts KL, Allen HA. Perception and cognition in the ageing brain: A brief review of the short- and long-term links between perceptual and cognitive decline. Front Aging Neurosci [Internet]. 2016;8:39. doi: https://doi.org/10.3389/fnagi.2016.00039
39. Fernandes DE, Kirsztajn GM, de Almeida K. Effect of hearing aids on attention, memory, and auditory evoked potentials: A pragmatic, single‐blinded, and randomised pilot clinical trial. Int J Clin Pract [Internet]. 2021;75(4):e13953. doi: https://doi.org/10.1111/ijcp.13953
40. Karawani H, Jenkins K, Anderson S. Restoration of sensory input may improve cognitive and neural function. Neuropsychologia [Internet]. 2018;114:203-13. doi: https://doi.org/10.1016/j.neuropsychologia.2018.04.041
41. Karawani H, Jenkins KA, Anderson S. Neural and behavioral changes after the use of hearing aids. Clin Neurophysiol [Internet]. 2018;129(6):1254-67. doi: https://doi.org/10.1016/j.clinph.2018.03.024
42. Johnson JA, Xu J, Cox RM. Impact of hearing aid technology on outcomes in daily life ii: Speech understanding and listening effort. Ear Hear [Internet]. 2016;37(5):529-40. doi: https://doi.org/10.1097/aud.0000000000000327
43. Wu YH, Stangl E, Chipara O, Hasan SS, DeVries S, Oleson J. Efficacy and effectiveness of advanced hearing aid directional and noise reduction technologies for older adults with mild to moderate hearing loss. Ear Hear [Internet]. 2019;40(4):805-22. doi: https://doi.org/10.1097/aud.0000000000000672
44. Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N, on behalf of the SENSE-Cog WP1 group. Longitudinal relationship between hearing aid use and cognitive function in older Americans. J Am Geriatr Soc [Internet]. 2018;66(6):1130-6. doi: https://doi.org/10.1111/jgs.15363
45. Dawes P, Emsley R, Cruickshanks KJ, Moore DR, Fortnum H, Edmondson-Jones M, et al. Hearing loss and cognition: The role of hearing aids, social isolation and depression. PLoS ONE [Internet]. 2015;10(3):e0119616. doi: https://doi.org/10.1371/journal.pone.0119616
46. Deal JA, Betz J, Yaffe K, Harris T, Purchase-Helzner E, Satterfield S, et al. Hearing impairment and incident dementia and cognitive decline in older adults: The health ABC study. J Gerontol A Biomed Sci Med Sci [Internet]. 2017;72(5):703-9. doi: https://doi.org/10.1093/gerona/glw069
47. Lunner T, Rudner M, Rönnberg J. Cognition and hearing aids. Scand J Psychol [Internet]. 2009;50:395-403. doi: https://doi.org/10.1111/j.1467-9450.2009.00742.x
48. Dupuis K, Pichora-Fuller MK, Chasteen AL, Marchuk V, Singh G, Smith SL. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Aging, Neuropsychology, and Cognition [Internet]. 2015;22(4):413-37. doi: https://doi.org/10.1080/13825585.2014.968084
49. Desjardins JL, Doherty KA. Age-related changes in listening effort for various types of masker noises. Ear Hear [Internet]. 2013;34(3):261-72. doi: https://doi.org/10.1097/aud.0b013e31826d0ba4
50. Wingfield A, Tun PA, McCoy SL. Hearing loss in older adulthood: What it is and how it interacts with cognitive performance. Current directions in psychological science [Internet]. 2005;14(3):144-8. doi: https://doi.org/10.1111/j.0963-7214.2005.00356.x
51. Akeroyd MA. Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. Int J Audiol [Internet]. 2008;47(Suppl 2):S53-71. doi: https://doi.org/10.1080/14992020802301142
52. Tye-Murray N, Spehar B, Myerson J, Hale S, Sommers M. Lipreading and audiovisual speech recognition across the adult lifespan: implications for audiovisual integration. Psychol Aging [Internet]. 2016;31(4):380-9. doi: https://doi.org/10.1037/pag0000094
53. Arlinger S. Negative consequences of uncorrected hearing loss-a review. Int J Audiol [Internet]. 2003;42(Suppl 2):17-20. doi: https://doi.org/10.3109/14992020309074639
54. Chen Y, Guan L, Chen J, Han K, Yu Q, Zhou J, et al. Hearing intervention for decreasing risk of developing dementia in elders with mild cognitive impairment: study protocol of a multicenter randomized controlled trial for Chinese Hearing Solution for Improvement of Cognition in Elders (CHOICE). Trials [Internet]. 2023;24:767. doi: https://doi.org/10.1186/s13063-023-07813-z
55. Chern A, Golub JS. Age-related hearing loss and dementia. Alzheimer Dis Assoc Disord [Internet]. 2019;33(3):285-90. doi: https://doi.org/10.1097/wad.0000000000000325
56. Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg [Internet]. 2014;150(3):378-84. doi: https://doi.org/10.1177/0194599813518021
57. Fu X, Liu B, Wang S, Eikelboom RH, Jayakody DMP. The relationship between hearing loss and cognitive impairment in a Chinese elderly population: The baseline analysis. Front Neurosci [Internet]. 2021;15:749273. doi: https://doi.org/10.3389/fnins.2021.749273
58. Slade K, Plack CJ, Nuttall HE. The effects of age-related hearing loss on the brain and cognitive function. Trends Neurosci [Internet]. 2020;43(10):810-21. doi: https://doi.org/10.1016/j.tins.2020.07.005
59. Raymond MJ, Lee AC, Schader LM, Moore RH, Raol NR, Vivas EX. Practices and perceptions of cognitive assessment for adults with age‐related hearing loss. Laryngoscope Investig Otolaryngol [Internet]. 2020;5(1):137-44. doi: https://doi.org/10.1002/lio2.339