Exertional Desaturation and Ambulatory Oxygen Therapy Requirements in People with Idiopathic Pulmonary Fibrosis. A Retrospective Study
Desaturación de esfuerzo y requerimientos de oxigenoterapia ambulatoria en personas con fibrosis pulmonar idiopática. Un estudio retrospectivo
Tamara Soler, Osvaldo Cabrera, Ruvistay Gutierrez-Arias , Francisca Lara, María Guacolda Benavides
Abstract
Introduction. Exertional desaturation (ED) is common in advanced idiopathic pulmonary fibrosis (IPF) stages. Ambulatory O2 therapy could increase physical activity by preventing ED in people with IPF.
Objective. This study aimed to assess ED and ambulatory O2 requirements in people with IPF using a protocol that involved up to four 6-minute walking tests (6MWT).
Method. An observational study of a dynamic retrospective cohort from a high-complexity hospital was conducted. The ambulatory O2 requirement assessment protocol involves performing up to four 6MWT depending on ED. All participants performed the baseline test (no additional O2). If ED (SpO2 < 90%) was observed, up to three additional 6MWTs were performed with two, four, and six O2 liters/minute until ED was avoided.
Results. Twenty-eight patients (16 female; mean age 73 years) were referred for assessment of ambulatory O2 requirements. Twenty-three (82%) had ED during baseline 6MWT. Twenty-two patients performed the 6MWT with two liters/minute of O2, ten performed a third 6MWT with four liters/minute of O2, and seven with six liters/minute of O2. The six participants who performed all four 6MWTs significantly increased their walking distance by 56.33 meters (SD 36.45) compared to the baseline (p = 0.001). Four of the seven patients (57.14%) who performed the last 6MWT had ED despite O2 supplementation.
Conclusion. The prevalence of ED during baseline 6MWT was high. Some participants even experienced ED with six liters/minute of supplemental O2. Despite this, walking distance increased more than the learning effect.
Keywords
Oxygen inhalation therapy; idiopathic pulmonary fibrosis; lung diseases; interstitial; walk test.
Resumen
Introducción. La desaturación de esfuerzo (DE) es frecuente en estadios avanzados de la fibrosis pulmonar idiopática (FPI). La terapia ambulatoria con O2 podría aumentar la actividad física al prevenir la DE en personas con esta enfermedad.
Objetivo. Este estudio tuvo como objetivo evaluar la DE y los requerimientos de O2 ambulatorio para evitar la DE en personas con FPI, utilizando un protocolo que incluyó hasta cuatro pruebas de caminata de 6 minutos (PC6min).
Método. Se realizó un estudio observacional de una cohorte retrospectiva dinámica en un hospital de alta complejidad. El protocolo de evaluación de los requerimientos de O2 ambulatorio implicó la realización de hasta cuatro PC6min dependiendo de la aparición de DE. Todos los participantes realizaron la prueba basal (sin O2 adicional). Si se observaba DE (SpO2 < 90%), se realizaban hasta tres PC6min adicionales con dos, cuatro y seis litros/minuto de O2 hasta evitar la DE.
Resultados. Veintiocho pacientes (16 mujeres; edad media de 73 años) fueron derivados para la evaluación de los requerimientos de O2 ambulatorio. Veintitrés (82%) presentaron DE durante la PC6min basal. Veintidós pacientes realizaron la PC6min con dos litros/minuto de O2, diez realizaron una tercera PC6min con cuatro litros/minuto de O2 y siete con seis litros/minuto de O2. Los seis participantes que realizaron las cuatro PC6min aumentaron significativamente su distancia caminada en 56,33 metros (DE 36,45) en comparación con el valor inicial (p = 0,001). Cuatro de los siete pacientes (57,14%) que realizaron la última PC6min presentaron DE a pesar de la suplementación con O2.
Conclusión. La prevalencia de DE durante la PC6min basal fue elevada. Algunos participantes incluso experimentaron DE con seis litros/minuto de O2 suplementario. A pesar de ello, la distancia recorrida aumentó por sobre el efecto de aprendizaje.
Palabras clave
Oxigenoterapia; fibrosis pulmonar idiopática; enfermedades pulmonares intersticiales; prueba de caminata de 6 minutos.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic respiratory condition characterized by the gradual fibrosis of the lung parenchyma [1]. The prevalence of this disease varies according to the definition used, ranging from 0.79 to 5.67 per 10,000 inhabitants in Asian, European, and North American countries [2]. In addition, the mortality reported in European Union countries between 2013 and 2018 was 3.9 per 100,000 person-years [3]. In Latin America, there are no epidemiological data on IPF. However, strategies such as the Latin American Registry of Idiopathic Pulmonary Fibrosis (REFIPI) could help to close this gap [4].
The IPF reduces lung compliance and gas diffusion [5], resulting in varying levels of hypoxemia, dyspnea, and exertional desaturation (ED). These phenomena reduce the functional capacity to exercise and the quality of life [6-9]. Because people with advanced stages of IPF experience desaturation at rest or during exertion, oxygen (O2) therapy is one of the main non-pharmacological treatments used for IPF patients. Exertional desaturation is an independent risk factor for increased mortality [10-12]. However, despite the uncertainty of its benefits, ambulatory O2 therapy is often indicated in people with IPF and ED [13,14].
Ambulatory O2 therapy could increase physical activity by correcting or preventing ED [15]. This may mitigate the limitations and restrictions experienced by people with IPF. However, the O2 level should be adjusted according to the individual's needs. Low levels may not resolve ED and promote the hypoxic pulmonary vasoconstriction reflex [16]. On the other hand, higher flows may lead to increased oxidative stress, a phenomenon linked to worsening IPF [17,18].
The British Thoracic Society's (BTS) guidelines for home O2 therapy in adults recommend ambulatory O2 therapy for home oxygen users who are mobile outdoors or for people undergoing an exercise or pulmonary rehabilitation program [19]. This guideline also states that some patients with interstitial lung disease could benefit from ambulatory O2 therapy [19]. However, the indication for this supplementary support should be based on a formal assessment of its benefit.
This study aimed to assess ED and ambulatory O2 requirements to avoid ED in people with IPF. The BTS protocol, which involved up to four 6-minute walking tests (6MWT), was adapted for this purpose [19]. Second, we assessed the variation in walking distance (6MWD) between different 6MWTs in patients who required supplemental O2 to avoid ED.
Method
Study design and setting
A retrospective observational dynamic cohort study was conducted. This study was based on clinical data extracted from medical records, so the waiver of informed consent was approved by the Servicio de Salud Metropolitano Oriente, Santiago de Chile, ethics committee on 17 October 2023 (SSMOriente17102023).
This study describes assessing the ambulatory O2 requirements of patients with IPF treated at the Instituto Nacional del Tórax between April 2020 and April 2023. The Instituto Nacional del Tórax is the Chilean national reference hospital managing complex cardiopulmonary diseases. All patients with IPF referred by bronchopulmonary physicians to evaluate ambulatory O2 prescriptions were considered.
Participants
Patients were retrospectively identified from the list of referrals to assess functional exercise capacity using the 6MWT. All adult patients (18 years and older) with IPF who underwent assessment of ambulatory O2 requirements using the 6MWT were included. The diagnosis of IPF was made by clinical, pathological, and radiological evaluation according to the international consensus of the American Thoracic Society/European Respiratory Society [20]. In addition, patients should have an arterial O2 pressure (PaO2) > 60 mmHg or a peripheral O2 saturation (SpO2) ≥ 90% at rest. Patients with any impairment in neuromusculoskeletal and movement-related function requiring any technical aid for ambulation (e.g., cane, crutches) were excluded.
Protocol for ambulatory oxygen therapy assessment
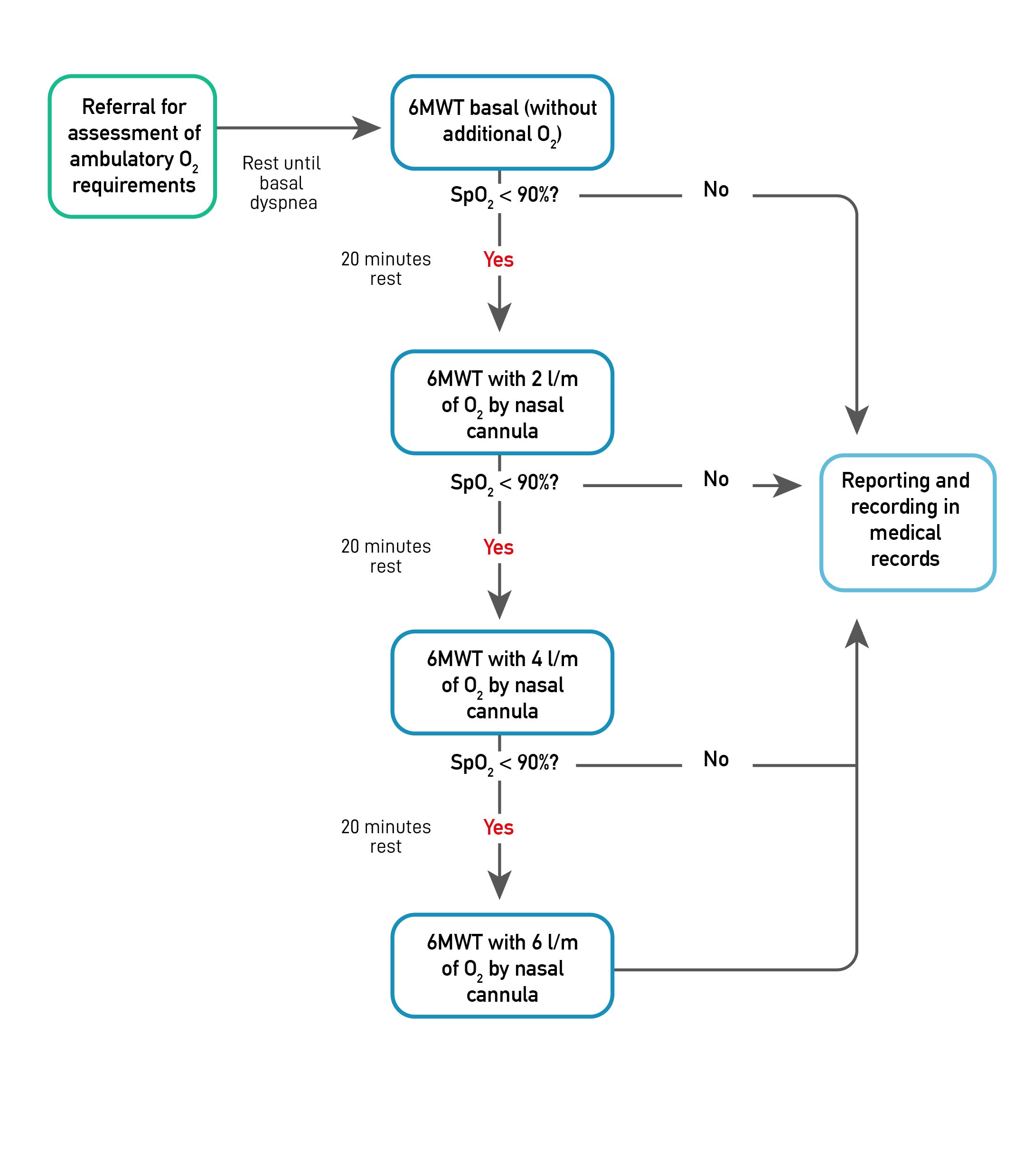
The ambulatory O2 requirement assessment protocol used is an adaptation of the one recommended by the BTS. It involves performing up to four 6MWTs depending on the presence of ED (SpO2 < 90%) [19]. The test proposed by the BTS is intended to determine the ED prevalence and, secondarily, to define the most appropriate device and O2 flow to avoid ED. Regarding this second objective, our protocol only considered different O2 flows administered with the D-type cylinder since it is our context's most widely available device.
The head physician of the home and ambulatory O2 program at the Instituto Nacional del Tórax revised and authorized the request from the bronchopulmonary physicians to assess the outpatient O2 requirements of IPF patients. Two physiotherapists applied the protocol to each patient, following the American Thoracic Society (ATS) guidelines for performing the 6MWT [21]. Before the first 6MWT (without additional O2), the patient rests in a chair until his dyspnea level returns to baseline. Then, before starting the initial test, heart rate (HR) (MASIMO®, Rad-5v), blood pressure (BP) (Biolight® BLT V6), respiratory rate (RR), SpO2 (MASIMO®, Rad-5v), and modified Borg scale were assessed. The HR, SpO2, and modified Borg scales were measured every minute during the test (Table 1).
Table 1. Variables measured in the four 6MWT.
| Variable | Evaluation timing |
|---|---|
| Heart rate, bpm | Every one minute during the test |
| Blood pressure, mmHg | At the beginning and end of the test |
| Respiratory rate, rpm | At the beginning and end of the test |
| Peripheral O2 saturation, % | Every one minute during the test |
| Dyspnea, modified Borg scale | Every one minute during the test |
If the patient's SpO2 dropped below 90% for at least 30 seconds, it was considered that the subject had ED and therefore required ambulatory O2. In this case, the O2 flow titration phase was continued. Otherwise, in the absence of ED, it was explained to the patient that it did not require ambulatory O2 and the protocol was finished.
The O2 flow titration phase began 20 minutes after the end of the basal 6MWT, at which time the patient's condition was checked to return to baseline. This phase consisted of performing a new 6MWT, with two liters/minute of O2 by conventional nasal cannula. If the ED was avoided with this O2 flow, the protocol was finished, and the results were explained to the patient. On the other hand, the titration protocol was continued if, despite the two liters/minute of O2, the ED persisted. The pattern described above was repeated for the following titration phases, considering 20 minutes of rest between each 6MWT and progressing to four and six liters/minute of O2 if ED prevailed (Figure 1). Patients were allowed to stop during the 6MWT, and physiotherapists stopped the test when SpO2 was less than 80% and with a Borg of 6 or greater [21].
Figure 1. Outpatient O2 requirements assessment protocol flowchart (adapted from BTS guideline [16]).
Note. 6MWT: six-minute walk test; SpO2: peripheral O2 saturation.
Data collection
Two physiotherapists collected data from participants who were eligible for this study. A standardized data extraction form was used, which was adjusted after being piloted in five patients. The data collected considered demographic and anthropometric characteristics at the time of undergoing the O2 assessment protocol (gender, age, weight, and height); diffusing capacity for carbon monoxide (DLCO); RR and BP before and after tests, HR, SpO2, and modified Borg during each of the 6MWT; and the 6MWD in each 6MWT.
The data were stored in an Excel spreadsheet and checked by a third physiotherapist. Each patient was assigned an identifier to anonymize the data.
Statistical methods
Categorical variables were summarized with absolute and relative values and quantitative variables were represented by mean with standard deviation (SD) or median with 25th and 75th percentile (IQR), depending on the distribution of each variable. The distribution of continuous variables was tested with the Shapiro-Wilk test.
To compare continuous variables with a normal distribution between two and three or more measurement times (BP, 6MWD, HR, and SpO2), students' t-tests and ANOVA for repeated measures were used, respectively. When the Mauchly sphericity assumption was violated, the Greenhouse-Geisser correction was used. Bonferroni’s test correction was used for the post-hoc comparison. In contrast, Mann-Whitney and Friedman's tests compared ordinal or non-parametric variables (RR and dyspnea) between two and three or more measurement times. Conover´s test correction was used for the post-hoc comparison.
All analyses considered a statistical significance level of 5% and were performed with the statistical program JASP -JASP Team 2022; JASP Version 0.16.3 (Computer software)-. The graphics were created using GraphPad Prism -Version 10.2.0 (392), © 1992-2024 GraphPad Software, LLC-.
Results
During April 2020 and April 2023, outpatient 1234 6MWTs were performed at the Instituto Nacional del Tórax. Among them, 28 patients (2.27%) with IPF were referred for assessment of ambulatory O2 requirements. The highest proportion was female (57.14%; Table 2).
Table 2. Characteristics of the study’s participants.
| Characteristics | All patients (n = 28) | No ED in 6MWT baseline (n = 5) | No ED in 6MWT with 2 lpm O2 (n = 12) | No ED in 6MWT with 4 lpm O2 (n = 4) | 6MWT with 6 lpm O2 (n = 7) |
|---|---|---|---|---|---|
| Female, n (%) | 16 (57.14) | 2 (40) | 8 (66.67) | 4 (100) | 2 (28.56) |
| Age (years), mean (SD) | 73 (8.40) | 74 (10.58) | 75.92 (4.36) | 71.25 (6.8) | 69.86 (13.72) |
| Height (cm), mean (SD) | 160.54 (10.68) | 168 (9.82) | 158.25 (9.42) | 149.25 (5.38) | 168.43 (10.96) |
| Weight (kg), mean (SD) | 69.21 (15.28) | 72,4 (12.18) | 65.83 (13.48) | 65 (14.85) | 81 (15.44) |
| PaO2 (mmHg), mean (SD) | 71.14 (14.82) | 69.25 (13.53) | 70.08 (17.8) | 62.5 (0.71) | 63.75 (0.18) |
| DLCO (% predicted), mean (SD) | 38.80 (11.23) | 41 (18.52) | 37 (10.11) | 34.5 (14.85) | 37.75 (13.2) |
Twenty-three (82.12%) patients had ED during baseline 6MWT. In one of them, the test had to be stopped at minute three due to desaturation up to 73% and a score of 8-9 on the modified Borg scale. Of the 22 patients who performed the 6MWT with two liters/minute of O2, 10 presented ED, for which they performed a third 6MWT with four liters/minute of O2. Six of these presented ED and, therefore, underwent a fourth performed 6MWT with six liters/minute of O2. The patient who did not complete the baseline 6MWT underwent a subsequent 6MWT with a six liters/minute O2 flow rate once they returned to their pre-assessment status (Figure 2). This participant was not included in assessing the variation in 6MWD and response to physical exertion (HR, SpO2, and dyspnea).
Figure 2. Patient selection flow chart.
Note. 6MWT: six-minute walk test.
At baseline 6MWT, systolic and diastolic BP increased significantly from a mean of 133.00 mmHg (SD 16.86) to a mean of 141.18 mmHg (SD 18.63; p = 0.015) and from a mean of 76.75 mmHg (SD 11.33) to a mean of 80.82 mmHg (SD 13.31; p = 0.015), respectively. In addition, RR increased significantly from a median of 30 bpm (IQR 24-36) to a median of 42 bpm (IQR 36-48; p < 0.001).
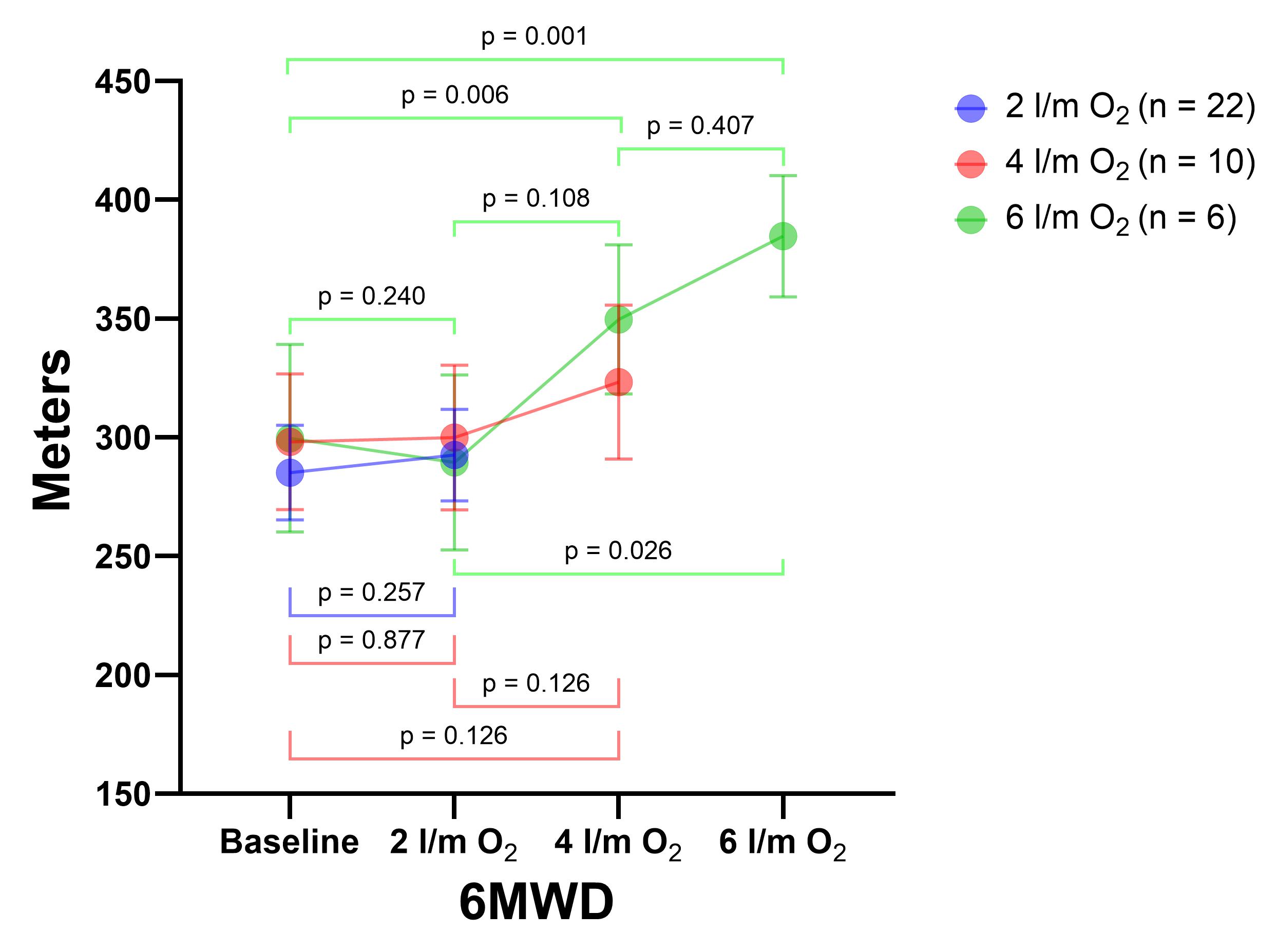
The mean 6MWD of the total participants at baseline was 288.4 (SD 109.29) meters. Patients who avoided ED on the 6MWT with two liters/minute of O2 increased their walking distance non-significantly by a mean of 7.36 meters (SD 29.66; p = 0.257). In addition, those who avoided ED with four liters/minutes of O2 walked more than those in the two previous 6MWTs; this difference was not statistically significant (p = 0.076). However, the six participants who performed all four 6MWTs significantly increased their walking distance by a mean of 56.33 meters (SD 36.45) more on six liters/minutes of O2 compared to the baseline test (p = 0.001; Figure 3). Four (57.14%) of the seven patients who performed the last 6MWT had ED despite O2 supplementation.
Figure 3. Comparison of walking distance (meters) between the 6MWT.
Note. For each test, participants who did not present ED were considered. The bars represent the standard error. 6MWD: Distance walked in 6MWT.
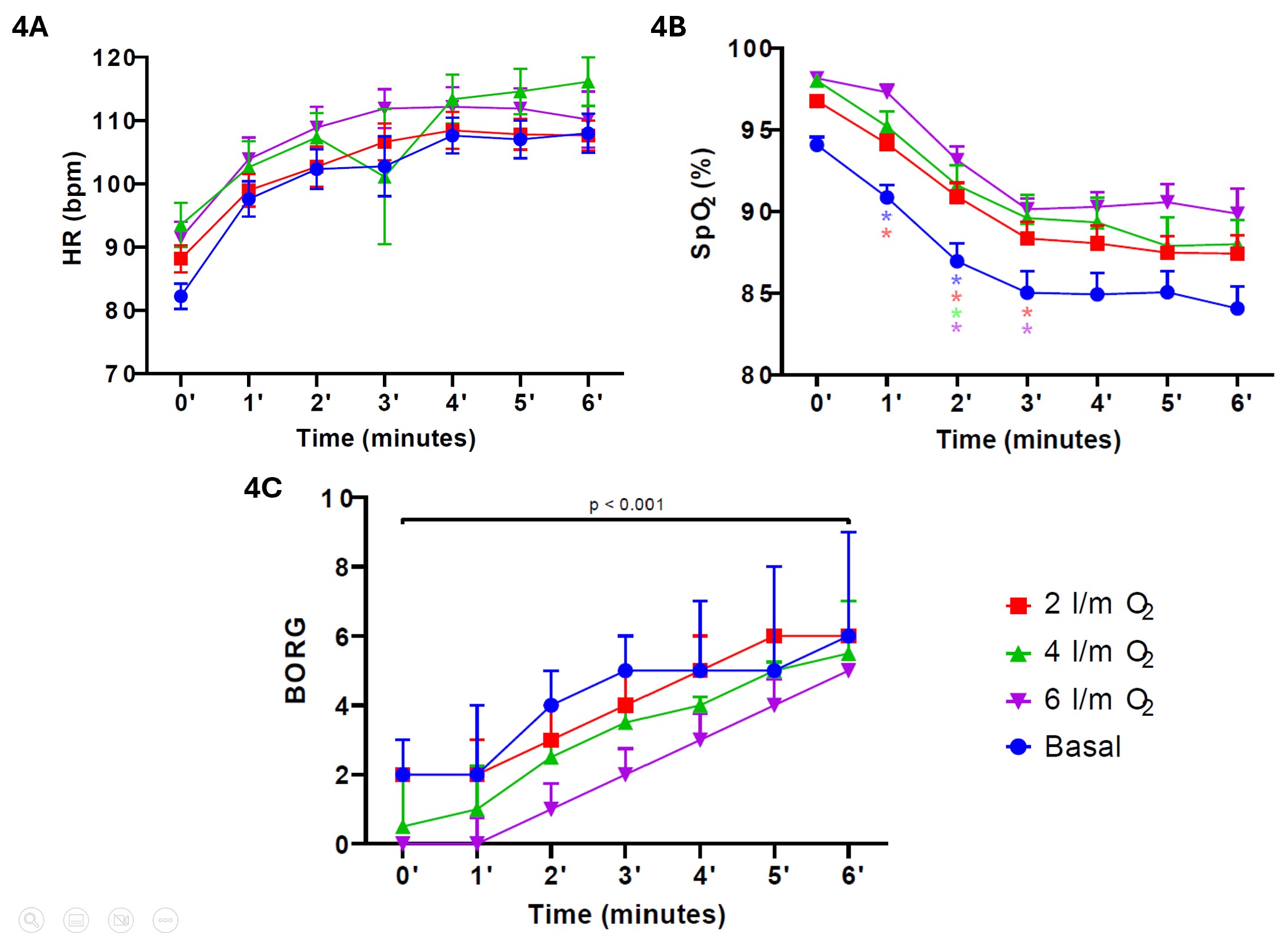
In the baseline 6MWT, the HR recorded in the first minute was, on mean, 14.5 bpm (SD 15.24) higher than at the start of the test (p < 0.001). However, the increase in HR from minute two onwards was insignificant (p > 0.05). This trend was similar in the other three 6MWTs (Figure 4A). In addition, at baseline 6MWT, SpO2 decreased significantly, on mean, by 3.07% (SD 3.97) at the first minute (p < 0.001) and 4.00% (SD 3.97) at the second minute (p < 0.001). Subsequently, SpO2 tended to decrease further; however, this desaturation was insignificant (p > 0.05). This trend was similar in the other three 6MWTs (Figure 4B). Regarding dyspnea, the modified Borg scale score at the end of all 6MWTs was significantly higher than the baseline (p < 0.001; Figure 4C).
Figure 4. Cardiorespiratory response (HR, SpO2, and modified Borg scale) during the 6MWTs.
Notes. 4A. HR response during the 6MWTs. 4B. SpO2 response during the 6MWTs. The colors of the marks (blue, red, green, and purple) refer to each 6MWT (basal, two l/m O2, four l/m O2, and six l/m O2). Marks indicate a significant difference (p < 0.05) from the previous minute. 4C. Dyspnea response during the 6MWTs. Bars represent standard error for HR and SpO2, and p25-75 for the modified Borg scale. HR: heart rate; bpm: beat per minute; SpO2: peripheral oxygen saturation.
Discussion
This observational study described a protocol to screen for ED and assess ambulatory O2 requirements in outpatients with IPF in a high-complexity hospital specialized in cardiac and respiratory diseases. This protocol, adapted from that proposed by the BTS [19], considers the performance of up to four 6MWTs, one baseline, and up to three additional 6MWTs with different flows of additional O2 via nasal cannula.
More than 80% of the participants in this study presented with ED. Exertional desaturation in patients with IPF is independently associated with survival rate [22]. Even though the evidence has shown no benefit other than reducing dyspnea and quality of life, ambulatory O2 is commonly prescribed to patients with IPF [23,24]. The most proposed test to screen for ED in patients with IPF is the 6MWT, which reflects people's usual daily activity. This test is reliable in this population [23-27].
Currently, there are strategies to titrate or predict ambulatory O2 flow by a single walking test [28,29]. The protocol proposed by Giovacchini et al. was found feasible and safe. However, the study population is heterogeneous, including patients with pathologies other than IPF [28]. During the walking test in the Giovacchini et al. study, participants went as far as walking for up to 15 minutes continuously to achieve stabilization of SpO2 > 88% for at least three minutes [28]. This requires less time than the full protocol proposed by the BTS [19]. However, Giovacchini's protocol does not allow us to determine whether adding supplemental O2 to prevent ED translates into other benefits, such as increased distance walking. On the other hand, the O2-GAP index is an algorithm created from the 6MWD of ILD patients that was shown to adequately predict O2 requirement for SpO2 >88% (r2 = 0.842; p < 0.001) [29]. However, based on the 6MWD predicted by the Enright and Sherill equations, which included healthy participants from Tucson, Arizona [30], it may only be applicable in some settings.
Our results are consistent with those reported by Bhatti et al., who included 155 patients with IPF in an exercise treadmill test, finding that 80% of patients with DLCO <50% had ED (SpO2 < 90%) [31]. However, the Khor et al. study reported a lower prevalence of ED detection by 6MWT in 214 (54%) of 400 patients with ILD [8]. This difference could be due to several factors. The population of Khor et al. study was more heterogeneous, including different subtypes of ILD, such as IPF, hypersensitivity pneumonitis, and connective tissue disease-related ILD [5], which could lead to a lower prevalence of ED. Furthermore, most of our patients had a DLCO < 50-55%, related to a higher prevalence of ED [8,31,32]. In addition, both Bhatti et al. and our study considered as a criterion for ED when patients had a SpO2 < 90% [31], whereas Khor et al. used a threshold of ≤ 88% [8]. In our study, we employed a SpO2 threshold of 90% to define ED, following the protocol established by the BTS [19]. However, there is no standard definition of ED. Besides using a different threshold (SpO2 ≤ 88%), other studies consider other criteria, such as a decrease of 4% or more in SpO2. Considering that the indication for ambulatory O2 is controversial in people with IPF, recommending this therapy, for example, to a person with a decrease in SpO2 from 96% to 92%, does not seem to be justified.
While our study showed that some participants persisted with ED despite two, four, or six liters/minutes of additional O2, 6MWD increased significantly in patients who performed all four 6MWTs. This difference could be attributed to the learning effect [33]. However, the six participants who performed the 6MWT with six liters/minutes of O2 walked 56.33 meters (SD 36.45) more than the baseline test, which exceeds the 21 meters (95% CI 12-30) attributed to the short-term learning effect in patients with IPF [26].
Our study has several limitations. First, the number of participants included was small, which may affect the accuracy of the results. Second, despite our hospital's strict record-keeping regarding the protocol's application, selection bias could not be fully managed as it was a retrospective study. Third, the DLCO of most patients in our study was below 50% (mean 38%), so we are still determining whether these results can be extrapolated to patients with less severe disease. Fourth, the maximum O2 flow used in our study was six liters/minute, conditioned by the interface (conventional nasal cannula). During 6MWT with this O2 supply, about 50% of the patients persisted with ED. This may have been because the patient's minute volume was higher than the O2 flow, making it impossible to meet ventilatory demands. Other O2 storage and interface systems should be used in future studies. Fifth, the protocol used in this study is challenging. However, none of the six participants who underwent the four 6MWTs failed to complete any tests, allowing assessment of cardiorespiratory response and walking distance performance. Reducing rest time according to patient tolerance and using shorter functional tests could increase the applicability of ED and O2 requirements assessment to avoid it.
Future prospective studies with more participants are needed to confirm our results. The application of our protocol is time-consuming, and modifications of the protocol should be evaluated.
Conclusions
Assessment of ambulatory O2 requirements of patients with IPF can be performed with a protocol that assesses ED using a baseline 6MWT and three with different levels of supplemental O2. Most of the participants in this study had ED during the baseline 6MWT. In addition, some participants even experienced ED with six liters/minute of supplemental O2. Despite this, 6MWD increased more than the learning effect. Future studies should confirm these results.
Applying this protocol in clinical practice is time-consuming, and it could challenge its implementation in settings with few professionals and limited infrastructure. Future studies should evaluate the use of other functional tests or modify the protocol proposed in this study to facilitate the assessment of ambulatory O2 prescriptions in people with IPF.
References
1. Wong AW, Ryerson CJ, Guler SA. Progression of fibrosing interstitial lung disease. Respir Res [Internet]. 2020;21(1):32. doi: https://doi.org/10.1186/s12931-020-1296-3
2. Maher TM, Bendstrup E, Dron L, Langley J, Smith G, Khalid JM, et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res [Internet]. 2021;22(1):197. doi: https://doi.org/10.1186/s12931-021-01791-z
3. Gonnelli F, Bonifazi M, Hubbard R. Mortality trends in idiopathic pulmonary fibrosis in Europe between 2013 and 2018. Eur Respir J [Internet]. 2024;64(2):2302080. doi: https://doi.org/10.1183/13993003.02080-2023
4. Caro F, Buendía-Roldán I, Noriega-Aguirre L, Alberti ML, Amaral A, Arbo G, et al. Latin American Registry of Idiopathic Pulmonary Fibrosis (REFIPI): Clinical Characteristics, Evolution and Treatment. Arch Bronconeumol [Internet]. 2022;58(12):794-801. doi: https://doi.org/10.1016/j.arbres.2022.04.007
5. Idiopathic Pulmonary Fibrosis: Diagnosis and Treatment. Am J Respir Crit Care Med [Internet]. 2000;161(2):646-64. doi: https://doi.org/10.1164/ajrccm.161.2.ats3-00
6. Janowiak P, Szymanowska-Narloch A, Siemińska A. IPF Respiratory Symptoms Management - Current Evidence. Front Med [Internet]. 2022;9. doi: https://doi.org/10.3389/fmed.2022.917973
7. Otake K, Misu S, Fujikawa T, Sakai H, Tomioka H. Exertional Desaturation Is More Severe in Idiopathic Pulmonary Fibrosis Than in Other Interstitial Lung Diseases. Phys Ther Res [Internet]. 2023;26(1):32-7. doi: https://doi.org/10.1298/ptr.E10218
8. Khor YH, Goh NS, Glaspole I, Holland AE, McDonald CF. Exertional Desaturation and Prescription of Ambulatory Oxygen Therapy in Interstitial Lung Disease. Respir Care [Internet]. 2019;64(3):299-306. doi: https://doi.org/10.4187/respcare.06334
9. Cox IA, Borchers Arriagada N, de Graaff B, Corte TJ, Glaspole I, Lartey S, et al. Health-related quality of life of patients with idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Eur Respir Rev [Internet]. 2020;29(158):200154. doi: https://doi.org/10.1183/16000617.0154-2020
10. Hallstrand TS, Boitano LJ, Johnson WC, Spada CA, Hayes JG, Raghu G. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur Respir J [Internet]. 2005;25(1):96-103. doi: https://doi.org/10.1183/09031936.04.00137203
11. Flaherty KR, Andrei A-C, Murray S, Fraley C, Colby TV, Travis WD, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med [Internet]. 2006;174(7):803-9. doi: https://doi.org/10.1164/rccm.200604-488OC
12. Hook JL, Arcasoy SM, Zemmel D, Bartels MN, Kawut SM, Lederer DJ. Titrated oxygen requirement and prognostication in idiopathic pulmonary fibrosis. Eur Respir J [Internet]. 2012;39(2):359-65. doi: https://doi.org/10.1183/09031936.00108111
13. Jacobs SS, Krishnan JA, Lederer DJ, Ghazipura M, Hossain T, Tan A-YM, et al. Home Oxygen Therapy for Adults with Chronic Lung Disease. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med [Internet]. 2020;202(10):e121-41. doi: https://doi.org/10.1164/rccm.202009-3608ST
14. Johannson KA, Pendharkar SR, Mathison K, Fell CD, Guenette JA, Kalluri M, et al. Supplemental Oxygen in Interstitial Lung Disease: An Art in Need of Science. Ann Am Thorac Soc [Internet]. 2017;14(9):1373-7. doi: https://doi.org/10.1513/AnnalsATS.201702-137OI
15. Arizono S, Furukawa T, Taniguchi H, Sakamoto K, Kimura T, Kataoka K, et al. Supplemental oxygen improves exercise capacity in IPF patients with exertional desaturation. Respirology [Internet]. 2020;25(11):1152-9. doi: https://doi.org/10.1111/resp.13829
16. Sommer N, Dietrich A, Schermuly RT, Ghofrani HA, Gudermann T, Schulz R, et al. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J [Internet]. 2008;32(6):1639-51. doi: https://doi.org/10.1183/09031936.00013908
17. Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med [Internet]. 2005;172(4):417-22. doi: https://doi.org/10.1164/rccm.200501-017PP
18. Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med [Internet]. 2009;103(9):1245-56. doi: https://doi.org/10.1016/j.rmed.2009.04.014
19. Hardinge M, Annandale J, Bourne S, Cooper B, Evans A, Freeman D, et al. British Thoracic Society guidelines for home oxygen use in adults: accredited by NICE. Thorax [Internet]. 2015;70:i1-43. doi: https://doi.org/10.1136/thoraxjnl-2015-206865
20. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med [Internet]. 2002;165(2):277-304. doi: https://doi.org/10.1164/ajrccm.165.2.ats01
21. Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J [Internet]. 2014;44(6):1428-46. doi: https://doi.org/10.1183/09031936.00150314
22. Vainshelboim B, Kramer MR, Izhakian S, Lima R, Oliveira J. Physical Activity and Exertional Desaturation Are Associated with Mortality in Idiopathic Pulmonary Fibrosis. J Clin Med [Internet]. 2016;5(8):1-11. doi: https://doi.org/10.3390/jcm5080073
23. Nishiyama O, Miyajima H, Fukai Y, Yamazaki R, Satoh R, Yamagata T, et al. Effect of ambulatory oxygen on exertional dyspnea in IPF patients without resting hypoxemia. Respir Med [Internet]. 2013;107(8):1241-6. doi: https://doi.org/10.1016/j.rmed.2013.05.015
24. Visca D, Mori L, Tsipouri V, Fleming S, Firouzi A, Bonini M, et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir Med [Internet]. 2018;6(10):759-70. doi: https://doi.org/10.1016/S2213-2600(18)30289-3
25. Eaton T, Young P, Milne D, Wells AU. Six-Minute Walk, Maximal Exercise Tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med [Internet]. 2005;171(10):1150-7. doi: https://doi.org/10.1164/rccm.200405-578OC
26. du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Six-Minute-Walk Test in Idiopathic Pulmonary Fibrosis: Test Validation and Minimal Clinically Important Difference. Am J Respir Crit Care Med [Internet]. 2011;183(9):1231-7. doi: https://doi.org/10.1164/rccm.201007-1179OC
27. Holland AE, Hill CJ, Dowman L, Glaspole I, Goh N, Lee AL, et al. Short- and Long-Term Reliability of the 6-Minute Walk Test in People With Idiopathic Pulmonary Fibrosis. Respir Care [Internet]. 2018;63(8):994-1001. doi: https://doi.org/10.4187/respcare.05875
28. Giovacchini CX, Mathews AM, Lawlor BR, MacIntyre NR. Titrating Oxygen Requirements During Exercise. Evaluation of a Standardized Single Walk Test Protocol. Chest [Internet]. 2018;153(4):922-8. doi: https://doi.org/10.1016/j.chest.2017.11.009
29. Ora J, Calzetta L, Pezzuto G, Senis L, Paone G, Mari A, et al. A 6MWT index to predict O2 flow correcting exercise induced SpO2 desaturation in ILD. Respir Med [Internet]. 2013;107(12):2014-21. doi: https://doi.org/10.1016/j.rmed.2013.10.002
30. Enrigth PL, Sherrill DL. Reference Equations for the Six-Minute Walk in Healthy Adults. Am J Respir Crit Care Med [Internet]. 1998;158(5-1):1384-7. doi: https://doi.org/10.1164/ajrccm.158.5.9710086
31. Bhatti W, Mamoor N, Kushnerick S, Chen X, Bon J, Gibson K, et al. Baseline Exertional Oxygen Needs in Patients with Idiopathic Pulmonary Fibrosis at a Specialty Referral Center. Chest [Internet]. 2020;158(Suppl 4):A1058-9. doi: https://doi.org/10.1016/j.chest.2020.08.979
32. Alfieri V, Crisafulli E, Visca D, Chong WH, Stock C, Mori L, et al. Physiological predictors of exertional oxygen desaturation in patients with fibrotic interstitial lung disease. Eur Respir J [Internet]. 2020;55(2):1901681. doi: https://doi.org/10.1183/13993003.01681-2019
33. Chandra D, Kulkarni HS, Sciurba F. Learning from the Learning Effect in the Six-Minute-Walk Test. Am J Respir Crit Care Med [Internet]. 2012;185(6):684. doi: https://doi.org/10.1164/ajrccm.185.6.684