The Effect of Difference Training Intensity on Increased Adiponectin Levels in High-fructose-induced Mice (Mus musculus)
El efecto de diferentes intensidades de entrenamiento sobre el aumento de los niveles de adiponectina en ratones (Mus musculus) inducidos por alta fructosa
Dwi Indah Puspita, Purwo Sri Rejeki , Gadis Meinar Sari, Misbakhul Munir, Nabilah Izzatunnisa, Muhammad, Shariff Halim, Adi Pranoto
Abstract
Introduction. The consumption of fructose in excessive quantities has been implicated in the onset of obesity and a spectrum of metabolic dysfunctions. Physical exercise is posited as a potent intervention to ameliorate obesity-induced metabolic anomalies, ostensibly through the elevation of adiponectin concentrations. However, the underlying molecular mechanisms of this effect remain inadequately understood.
Objective. This study aims to demonstrate the impact of exercise intensity on increasing adiponectin levels in high-fructose-induced mice, highlighting the underlying molecular mechanisms.
Methods. The experiment was carried out on 36 male mice (Mus musculus), aged ±8 weeks, with body weight ± 20 - 25 grams, in healthy condition and without defects. Mice were randomly divided into four groups. Control group without training (CN; n = 9); the low-intensity swimming training group with a 3% load of the mice's body weight (LI; n = 9); the moderate-intensity swimming training group with a 6% load of the mice's body weight (MI; n = 9); the heavy intensity swimming training group with a 9% load of the mice's body weight (HI; n = 9). The frequency of swimming training was carried out 3 times/week for 8 weeks, and the duration of swimming training was calculated as 80% of the maximum swimming time every session. All groups were orally (oral ad libitum) given 30% fructose solution for 8 weeks. Adiponectin levels were quantified via ELISA. Statistical interrogation employed one-way ANOVA and Tukey's HSD post hoc test, with a significance threshold set at 5%.
Results. The results indicated a statistically significant divergence in adiponectin levels (p ≤ 0.001). Tukey's HSD post hoc test analysis revealed substantial disparities between CN and LI (p = 0.196), CN and MI (p = 0.0001), CN and HI (p = 0.001), LI and MI (p = 0.001), LI and HI (p = 0.001), and MI and HI (p = 0.001).
Conclusion. This study found that moderate-intensity swimming training was more optimal in increasing adiponectin levels in fructose-induced mice compared to high-intensity, low-intensity, and control groups. Additionally, this research identified specific molecular pathways activated by moderate-intensity training, providing new insights for therapeutic interventions in tackling obesity-related metabolic dysfunctions.
Keywords
High-fructose corn syrup; swimming training; receptors, adiponectin; obesity.
Resumen
Introducción. El consumo excesivo de fructosa se ha asociado con la aparición de la obesidad y una serie de disfunciones metabólicas. Se postula que el ejercicio físico es una intervención potente para mejorar las anomalías metabólicas inducidas por la obesidad, aparentemente a través del aumento de las concentraciones de adiponectina. Sin embargo, los mecanismos moleculares subyacentes a este efecto siguen sin comprenderse adecuadamente.
Objetivo. Este estudio tiene como objetivo demostrar el impacto de la intensidad del ejercicio en el aumento de los niveles de adiponectina en ratones inducidos por alto contenido en fructosa, destacando los mecanismos moleculares subyacentes.
Métodos. El experimento se realizó en 36 ratones machos (Mus musculus), de ±8 semanas de edad, con un peso corporal de ± 20 - 25 gramos, en condiciones saludables y sin defectos. Los ratones se dividieron aleatoriamente en cuatro grupos. El grupo control sin entrenamiento (CN; n = 9); el grupo de entrenamiento de natación de baja intensidad con una carga del 3% del peso corporal de los ratones (LI; n = 9); el grupo de entrenamiento de natación de intensidad moderada con una carga del 6% del peso corporal de los ratones (MI; n = 9); y el grupo de entrenamiento de natación de alta intensidad con una carga del 9% del peso corporal de los ratones (HI; n = 9). La frecuencia del entrenamiento de natación se llevó a cabo 3 veces/semana durante 8 semanas, y la duración del entrenamiento de natación se calculó como el 80% del tiempo máximo de natación en cada sesión. Todos los grupos recibieron una solución de fructosa al 30% por vía oral (ad libitum) durante 8 semanas. Los niveles de adiponectina se cuantificaron mediante ELISA. El análisis estadístico se realizó mediante ANOVA de una vía y la prueba post hoc de Tukey HSD, con un umbral de significancia establecido en 5%.
Resultados. Los resultados indicaron una divergencia estadísticamente significativa en los niveles de adiponectina (p ≤ 0.001). El análisis post hoc de Tukey HSD reveló diferencias sustanciales entre CN y LI (p = 0.196), CN y MI (p = 0.0001), CN y HI (p = 0.001), LI y MI (p = 0.001), LI y HI (p = 0.001), y MI y HI (p = 0.001).
Conclusión. Este estudio encontró que el entrenamiento de natación de intensidad moderada fue más óptimo para aumentar los niveles de adiponectina en ratones inducidos por fructosa en comparación con los grupos de alta intensidad, baja intensidad y control. Además, esta investigación identificó vías moleculares específicas activadas por el entrenamiento de intensidad moderada, proporcionando nuevas perspectivas para intervenciones terapéuticas en la lucha contra las disfunciones metabólicas relacionadas con la obesidad.
Palabras clave
Jarabe de maíz con alto contenido de fructosa; entrenamiento de natación; receptores, adiponectina; obesidad.
Introduction
Obesity and overweight are considered global health burdens related negatively to quality of life, such as increased morbidity and mortality [1]. Obesity is now the fifth-ranked risk factor for death worldwide [2]. Globally, adults with a body mass index ≥ 25 kg/m2 increased from 28.8% for men and 29.8% for women in 1980, then increased to 36.9% for men and 38% for women in 2013 [3]. Based on the current pattern, the rates of obesity will reach 18% for men and 21% for women by 2025, and 1 in 5 women and 1 in 7 men will be living with obesity, which will affect approximately 1 billion people globally by 2030 [4]. Meanwhile, in Indonesia, the obesity prevalence rate increased from 10.3% in 2007 to 15.4% in 2013 [5].
Fructose is the most used sweetener in the food industry, and the high consumption of fructose is strongly associated with the onset of obesity [6]. Fructose is more palatable and less promote satiety, which caused an increase in the food consumption and disrupts the metabolism of carbohydrates and lipids, thus promoting fat synthesis and accumulation in adipose tissue [7]. Adipose tissue is an endocrine organ that can respond to incoming signals and secrete several peptide hormones [8-9]. Adiponectin is a hormone secreted by adipose tissue and functions to protect several pathological events in various cells by suppressing cell death, inhibiting inflammation, and increasing cell survival [10]. Obese and type 2 diabetic subjects have low adiponectin levels, due to impair function in adipose tissue hyperplasia and hypertrophy state [11-12]. This is because adiponectin expression is inhibited by hypoxia, oxidative stress [13], insulin resistance, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) [14]. Adiponectin levels will increase with weight loss and increased insulin sensitivity [15].
Exercise has been widely recommended as an effective therapeutic strategy to prevent many obesity-related metabolic disorders [16-18]. The swimming intervention might reduce body weight, visceral mass, and hepatic lipid accumulation. Also, it might improve insulin resistance and inflammatory state [19]. Adiponectin levels were increased by exercise and were inversely related with adipocyte mass, due to adiponectin controlling metabolism through blood glucose and fatty acid oxidation [20-21]. This makes adipose tissue more sensitive to the effects of Fibroblast Growth Factor 21 (FGF21), which promotes glucose uptake by insulin, regulates lipolysis, induces adiponectin secretion, and increases mitochondrial biogenesis [22-24]. The increase in adiponectin results from exercise is expected to increase protection against several pathological events in various cells.
In addition to adiponectin levels, other variables such as the Lee Index and Fasting Blood Glucose (FBG) are critical indicators of metabolic health in obesity research. The Lee Index, a measure of obesity in rodents, provides a useful comparative tool for assessing the impact of exercise on body composition [25]. FBG levels are a direct indicator of glucose metabolism and insulin sensitivity, which are crucial for understanding the metabolic effects of exercise interventions [26]. These variables are essential for providing a comprehensive analysis of how exercise intensity influences metabolic health in fructose-induced obese mice.
Recent research has examined how exercise interventions tested in rodent models can be applied to humans. For example, previous research found that moderate-intensity exercise led to comparable metabolic improvements in both rodent models and human clinical trials, highlighting the importance of these preclinical studies for developing human treatments [27]. Similarly, another study found that the molecular pathways triggered by exercise in mice are also present in humans, indicating that animal studies can offer valuable insights into human physiology and guide therapeutic approaches [28].
While there are existing studies on the effect of interval training on adiponectin levels using similar exercise protocols in mice fed with fat and fructose, this study distinguishes itself by focusing specifically on the differential impact of exercise intensities (low, moderate, and high) on adiponectin levels, Lee Index, and FBG in high-fructose-induced obese mice. This approach allows for a detailed analysis of the optimal exercise intensity for maximizing the protective effects of adiponectin against metabolic dysfunctions. By elucidating the underlying molecular mechanisms, this research aims to fill the gap in knowledge regarding how specific exercise intensities can be utilized as therapeutic strategies to address obesity-related metabolic disorders.
However, the molecular mechanisms underlying this effect remain poorly understood. Therefore, this study aims to demonstrate the diferential impact of exercise intensity on increasing adiponectin levels in high-fructose-induced mice, with a focus on elucidating the underlying molecular mechanisms. Despite the established effects of exercise on fructose metabolism, the translational application of these findings from animal models to humans necessitates careful consideration due to physiological differences. We can better predict how such interventions might translate to human physiology and inform the development of more effective therapeutic strategies, as a result of understanding these mechanisms. By elucidating the underlying molecular mechanisms, the research also highlights the potential of moderate-intensity exercise as a therapeutic strategy to address obesity-related metabolic disorders, with a focus on improvements in insulin sensitivity and glucose metabolism.
Material and Methods
Research design
This was a true-experimental study with a randomized posttest-only control group design. The experiment was carried out on 36 male mice (Mus musculus), aged ±8 weeks, with body weight of ± 20 - 25 grams, in healthy condition and without defects. Acclimatization was carried out for seven days before the study by being placed at room temperature 26 ± 2°C with humidity 50-60% [29]. The food pellet (BR-1, PT Japfa Comfeed, Indonesia Tbk) was given at 08.00, and drinks containing 30% fructose solution were given ad libitum for 8 weeks. BR-1 contained 21% protein, 3-7% fat, 0.9-1.1% calcium, and 0.6-0.9 phosphor. The fructose solution was freshly made every day. Food was given at 08.00, and drinks containing 30% fructose solution were given ad libitum during 8 weeks. Mice were randomly divided into four groups. The control group without training (CN; n = 9); the low-intensity swimming training group with a 3% load of the mice's body weight (LI; n = 9); the moderate-intensity swimming training group with a 6% load of the mice's body weight (MI; n = 9); the heavy-intensity swimming training group with a 9% load of of the mice's body weight (HI; n = 9). All groups were given 30% fructose solution orally (oral ad libitum) for 8 weeks. This experimental study was approved by the Health Research Ethics Committee, Faculty of Medicine, Airlangga University, Surabaya No. 153/EC/KEPK/FKUA/2022.
Exercise protocol
The exercise protocol was done according to the previous study with slight modification [30]. The training given for mice for each group was swimming. The control group (CN) was not given any training, LI was given swimming training with a 3% load of the mice's body weight, MI was given swimming with a 6% load of the mice's body weight, and HI was given swimming with a 9% load of the mice's body weight [31]. The training time began with adaptation of mice to the water for seven days. Briefly, the fresh water was filled in the water tanks (diameter: 61 cm; height 22.5 cm), and the water temperature was maintained at 32°C to prevent hypothermia [32]. The acclimatization procedure for mice is carried out by swimming in a water tank for 10 minutes without using weights, then increased 10 min daily until mice could swim for 30 min [33]. The maximum time was recorded when the mouse sinks once and releases air bubbles. The different load was expected to balance energy through improving physical activity, lipid profile, and decreasing the visceral fat accumulation [34-35]. The frequency of swimming training was carried out 3 times/week for 8 weeks, every time at 16.00. The duration of swimming training per session was calculated as 80% of the maximum swimming time using weights according to the training intensity of each group. Durations of low-, moderate-, and heavy-intensity training were 15 minutes, 12.5 minutes, and 10 minutes, respectively.
Anthropometric measurements and analysis of biochemical
Body weight and body lenght were measured using a digital scale Camry EK3250 toolbar (torsion balance; 0-5 kg) and iron ruler pre- and post-treatment for eight weeks. The fasting blood glucose levels were measured twice using glucometer at pre- and post-treatment. Measurements of Lee’s index pre- and post-treatment for 8 weeks were carried out using the cube root formula of body weight in grams divided by naso-anal length in mm and multiplied by 10,000 [36]. The mice were sacrificed and 1 ml of blood was taken from the mice's left ventricle 12 hours after the last swimming exercise in fasting conditions. The blood was centrifuged for 15 minutes at a speed of 3000 rpm. The serum was separated and stored at -80°C for analysis of adiponectin levels the following day. Adiponectin levels were measured using the Enzyme-Linked Immunosorbent Assay (ELISA) Kits (Cat.No:E-EL-M0002; Elabscience Biotechnology Co. Ltd., USA). The accuracy of our ELISA used has been validated by previous studies [37].
Statistical analysis
Statistics were calculated using the Statistical Packet for Social Science (SPSS) software version 21; quantitative data were expressed as mean ± standard deviation (mean ± SD). Data were analyzed using normality and homogeneity tests. The bodyweight and fasting blood glucose before and after training was analyzed using a paired sample t-test. The adiponectin levels, delta (∆) of weight, body length, lee index, and fasting of blood glucose (FBG) between groups were analyzed using the one-way-ANOVA test, and then continued with Tukey's Honestly Significant Difference (HSD) post hoc test. Meanwhile, effect size evaluation was applied with Cohen’s d. The relationship between the dependent variable with the independent variable were analyzed using the Pearson's product moment correlation coefficient. A p-value ≤ 0.05 was considered to have a significant difference.
Results
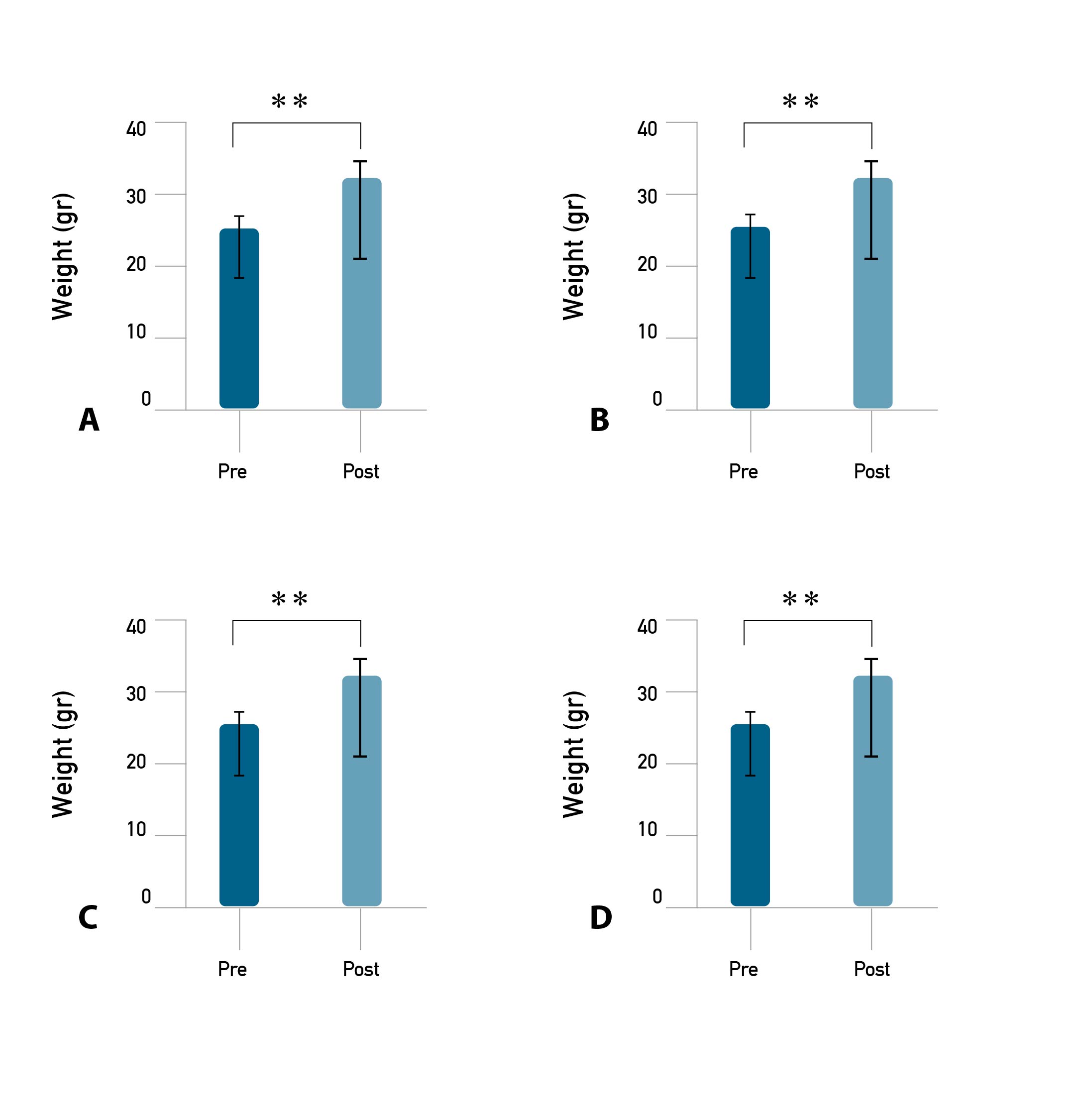
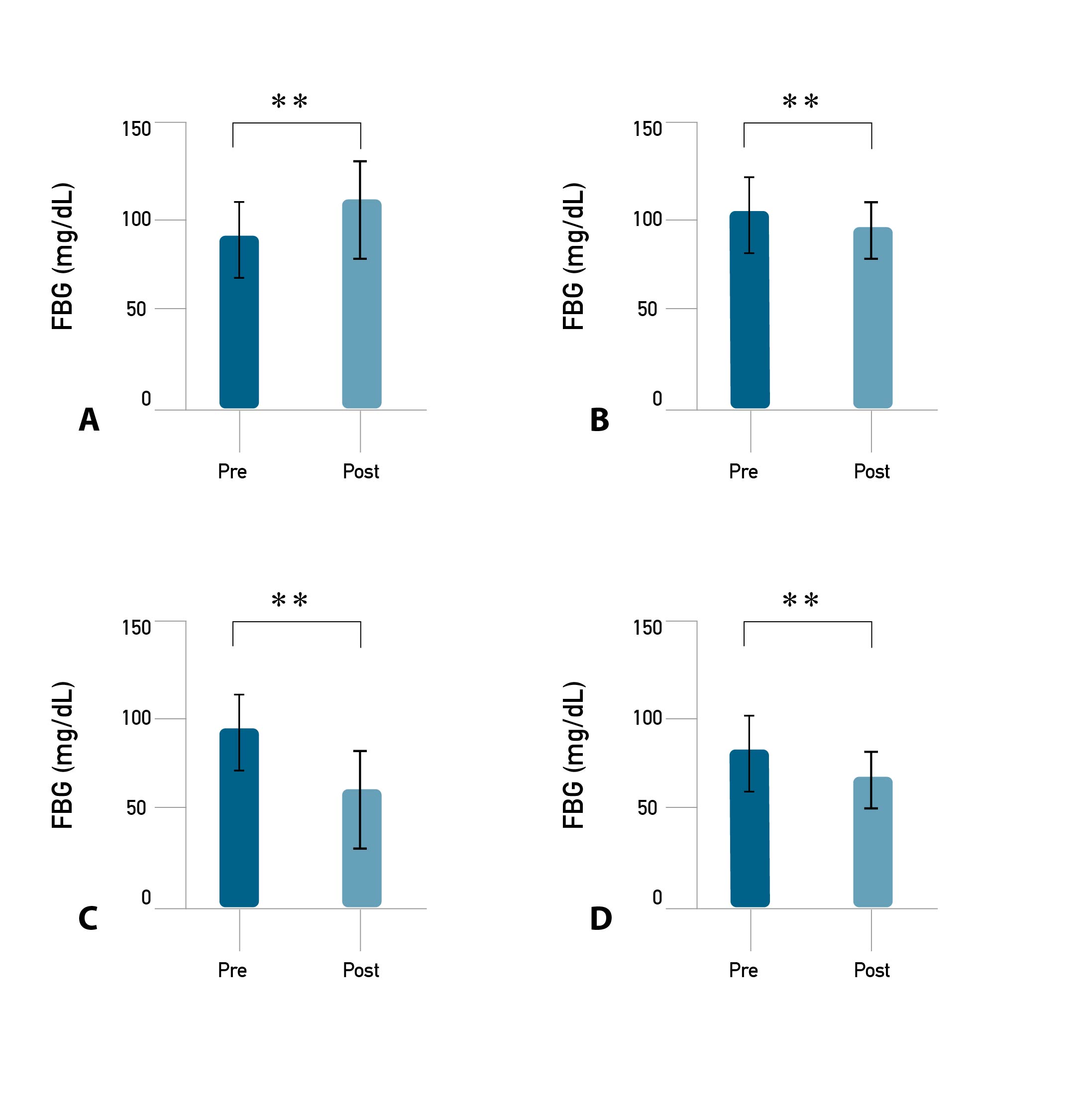
The results of the study showed that there was a change in the average body weight of mice between pre- vs. post-training for eight weeks. The results of the paired sample t-test show that there was a significant difference in the average body weight of mice between the pre- vs. post-training on C, LI, MI, and HI (p ≤ 0.001). On average, the highest increase in body weight between post-training in C, followed by LI, HI, and the lowest increase occurred in MI (Figure 1). The results of the paired sample t-test showed that there was a significant difference in average fasting of blood glucose (FBG) between pre- vs. post-training for eight weeks in each group (p ≤ 0.001). The highest decrease in FBG post-training occurred in MI, followed by HI, LI, and CN increased (Figure 2). Meanwhile, different delta (∆) of weight, body length, lee index, and FBG between groups (CN vs LI vs MI vs HI) are presented in Table 1.
Figure 1. Body weight pre- and post-exercise in each group.
Note. Description: (A) Control group (CN); (B) Low-intensity training group (LI); (C) Moderate-intensity training group (MI); (D) Heavy intensity training group (HI). (**) Significantly different from pre (p ≤ 0.001).
Figure 2. Fasting of blood glucose (FBG) pre- and post-exercise in each group.
Note. Description: (**) Significantly different from pre (p ≤ 0.001).
Table 1. Different delta (∆) of weight, body length, lee index, and FBG between groups (CN vs LI vs MI vs HI).
| Parameters | CN (n=9) | LI (n=9) | MI (n=9) | HI (n=9) | p-Value |
|---|---|---|---|---|---|
| ∆-Weight (gr) | 10.22±2.86 | 9.33±1.73 | 4.89±1.90*^ | 6.11±4.46* | 0.001 |
| ∆-Body length (cm) | 1.37±0.58 | 0.58±0.36* | 1.13±0.35 | 0.82±0.67 | 0.013 |
| ∆-Lee Index (gr/mm) | -0.01±0.02^ | 0.02±0.01 | -0.02±0.02^ | -0.01±0.03 | 0.002 |
| ∆-FBG (mg/dL) | 17.33±8.43 | -9.11±8.98* | -30.22±19.95*^ | -21.11±14.83* | 0.000 |
Nota. Description: (*) Significantly different from CN (p ≤ 0.05). (^) Significantly different from LI (p ≤ 0.05)
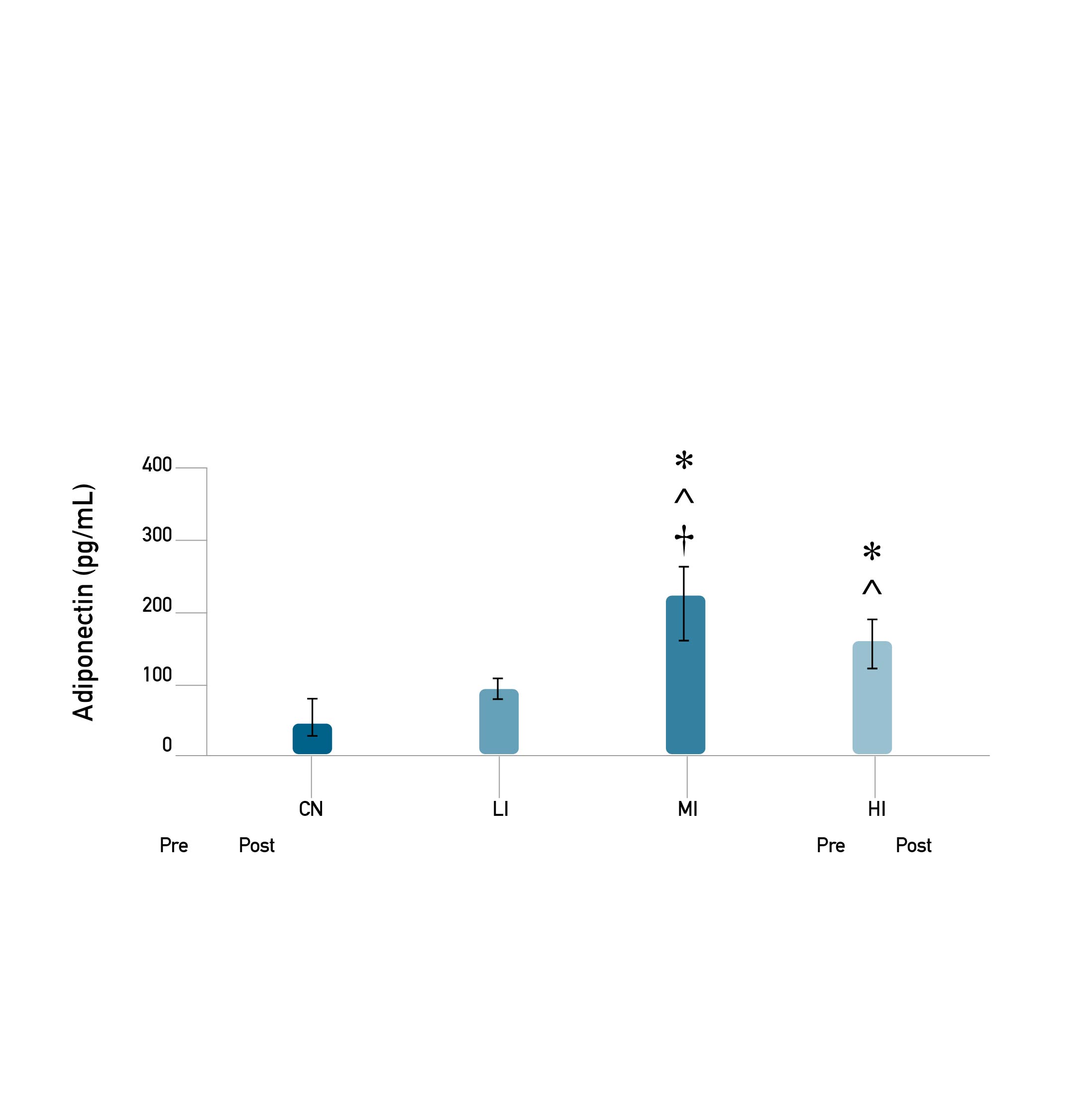
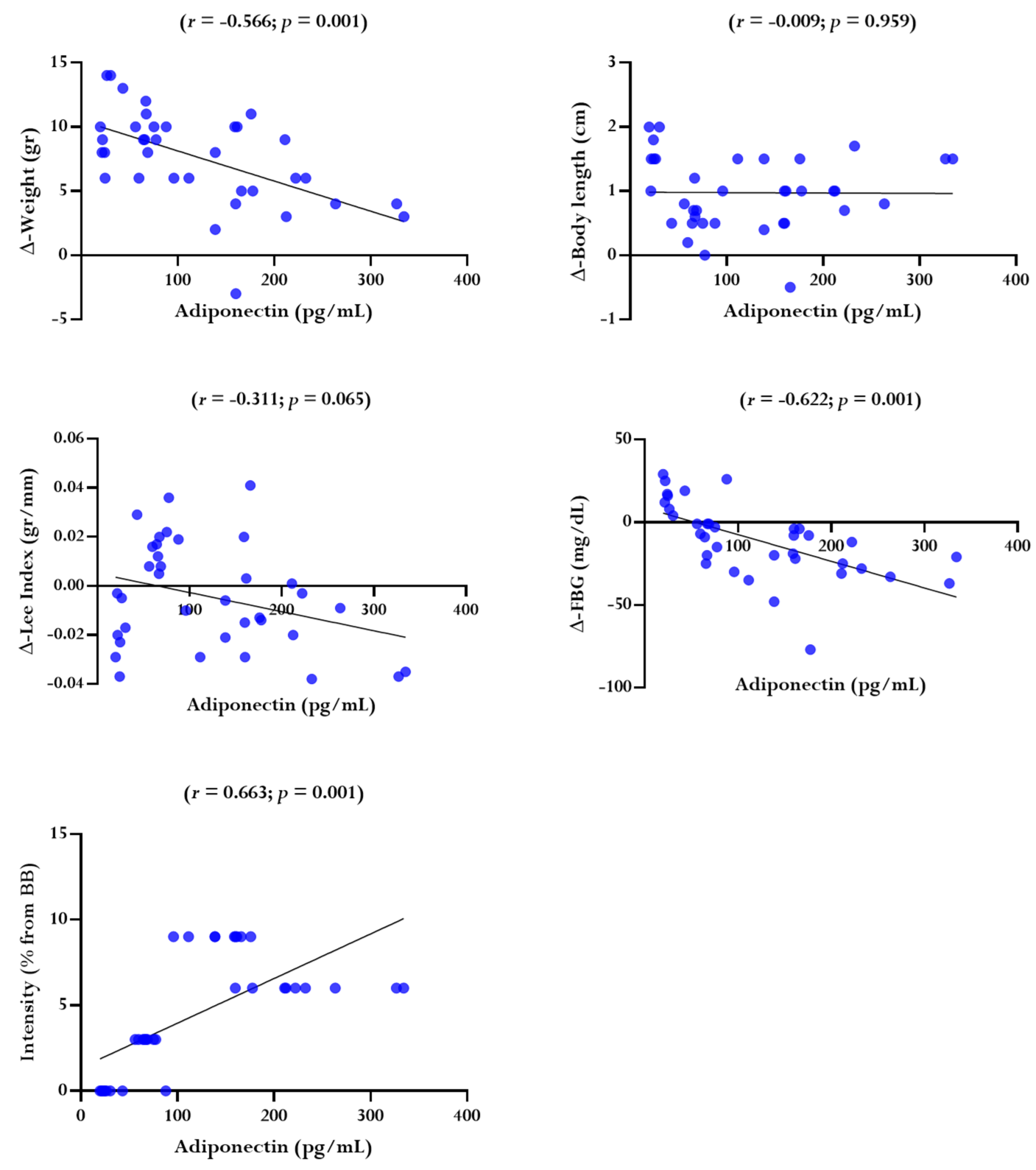
The results of the descriptive analysis of the average adiponectin levels in between groups were highest in MI, followed by HI and LI, and the lowest occurred in CN. The results of the normality and homogeneity tests show that the data was normally distributed and has homogeneous variance (p ≥ 0.05). The results of the one-way ANOVA test on average adiponectin levels showed that there was a significant difference in average adiponectin levels (p ≤ 0.001) (Figure 3). Based on the results of Tukey's HSD post hoc test, there was a significant difference in average adiponectin levels between CN with LI (p = 0.196); MI with CN (p = 0.0001) and there was a large effect size with a Cohen's d value of 4.504; HI with CN (p = 0.001) and there was a large effect size with a Cohen's d value of 4.612; MI with LI (p = 0.001) and there was a large effect size with a Cohen's d value of 3.973; HI with LI (p = 0.001) and there was a large effect size with a Cohen's d value of 4.028; MI with HI (p = 0.001) and there was a large effect size with a Cohen's d value of 1.979 (Figure 3). Meanwhile, the relationship between the dependent variable with the independent variable are presented in Figure 4.
Figure 3. Analysis of the average adiponectin levels in between group.
Note. Description: (*) Significantly different from CN (p ≤ 0.001). (^) Significantly different from LI (p ≤ 0.001). (†) Significantly different from HI (p ≤ 0.001).
Figure 4. The relationship between the dependent variable with the independent variable.
Note. Description: r and p -values were obtained using the Pearson's product moment correlation coefficient test.
Discussion
The present study explained the long-term exercise effects on the body weight, fasting blood glucose, and adiponectin levels in mice with high fructose diet. The exercise duration is the same standard with treadmill exercise, that when performed equal or greater than 6 weeks is categorized as a long-term duration [38]. The results of this study showed that all intervention groups had the same effect on increasing body weight in mice (Figure 1). This aligns with Rahayu et al. [39], confirming that various intensities of exercise, regardless of type, lead to weight gain in a high-fructose diet context. Similar to our findings, the lowest weight gain occurred in the moderate-intensity group, while the highest weight increase occurred in the control group. The significant body weight increasement in the control group (C), different from the other groups, was due to an imbalance between calories intake and calories expenditure, causing excessive accumulation of fat tissue in the body, as well as an increase in body weight [40]. Subcutaneous and visceralfat are the primary energy sources for muscle contraction during exercise. The fatty acid oxidation takes the energy from fat tissue storage, which leads to the reduction of fat mass following by the body weight decrease [41], causing the weight of mice in the exercise group to be lower compared to the control group (C).
Recent studies have highlighted the translational potential of such findings from animal models to humans. Beck and Meyerholz [27] reported that moderate-intensity exercise in rodent models led to metabolic improvements comparable to those observed in human clinical trials, underlining the translational relevance of preclinical studies. Additionally, Roberts et al. [28] identified conserved molecular pathways activated by exercise in both mice and humans, suggesting that the mechanisms observed in animal studies may be applicable to human physiology.
Our result demonstrated that there is a decrease in fasting of blood glucose levels during exercise (Figure 2 A-D). A previous study reported that decrease in blood glucose is the improvement sign of insulin secretion and lipolysis [42]. Excess calories in the body will be converted into glucose and stored in the liver in the form of glycogen [43]. Glycogen levels in the liver have certain optimal limits so that if blood glucose levels are sufficient, while glucose intake continues, a mechanism for converting glucose into fat occurs, which will be stored in adipose tissue in the form of fat pads [43]. However, in the present study, we did not measure the sucrose and appetite of mice. Thus, we cannot be certain of any relationship between exercise and adiponectin. Further work is needed to measure the appetite of mice and compare it with adiponectin levels and its receptor, as other adipokine such as leptin or ghrelin might be important.
Based on the adiponectin levels in Figure 3, the moderate-intensity group (MI) has the highest levels, followed by the heavy-intensity group (HI), the low-intensity group (LI), and the control group (CN). The higher levels of adiponectin in the exercise group compared to controls might due to the improvement of adipose tissue. The previous study reported that exercise induces PPAR-γ activation, which promotes glucose uptake by insulin and induces adiponectin secretion [24,44]. The results of this study are similar to the study of Zelikovich et al. [21], who used samples of mice with muscular dystrophy that were treated with aerobic exercise (running on a treadmill) for 24 weeks. The results of this study stated that adiponectin levels were higher in the moderate-intensity group compared to the low-intensity group and controls. Also, the same results were found in muscle adiponectin levels, which showed that the moderate-intensity training group was higher than the high-intensity training group. This can be caused because adiponectin is not only produced by fat cells, but also by muscle cells and can act in an autocrine/paracrine manner [45]. Adiponectin was increased during aerobic exercise in fat and systemic circulation, which in turn decreased the adipocyte volume [21].
Interestingly, the results of our study showed that MI exercise (swimming) had a higher effect on weight loss and increased serum adiponectin levels compared to HI exercise. A previous study reported that adiponectin signaling was stronger in the MI training than in HI training. This is due to the MI training induced fat oxidation, which is stimulated by higher levels of adiponectin production in the muscle. Additionally, the different training intensity seems to affect the muscle adiponectin production [46]. In contrast, HI demonstrated to improve proinflammatory marker, such as TNF-α and neurotrophic factor in the brain than MI which is resulting in a positive effect in physical activity [47].
A critical analysis may involve exploring the mechanisms behind why moderate-intensity exercise showed superior effects on adiponectin levels compared to other intensities. One possible explanation is the balance between energy expenditure and metabolic adaptations induced by moderate exercise intensity, leading to optimal adiponectin secretion and metabolic regulation. Moderate-intensity exercise may provide a balanced energy expenditure that avoids excessive stress on the body, promoting a favorable environment for adiponectin production. Furthermore, the molecular pathways involved in exercise-mediated adiponectin production, such as the activation of PPAR-γ and the enhancement of fatty acid oxidation, contribute to improved glucose metabolism and reduced inflammation. These pathways may play a significant role in the superior effects observed with moderate-intensity exercise [48-49].
The findings of this study underscore the potential of moderate-intensity as a therapeutic strategy for combating obesity-related metabolic disorder. The significant increase in adiponectin levels observed in the moderate-intensity group highlights the role of this hormone protecting against various pathological condition. Furthermore, adiponectin's involvement in improving insulin sensitivity and glucose metabolism suggests that moderate-intensity exercise can be particularly beneficial in managing obesity and its associated health risks. The difference result might be from the different animal model used and the exercise training set during treatment. Further studies are required to elucidate the detailed mechanism about how different exercise-load might be beneficial for improving muscle adiponectin under obese conditions. The limitations of the study are that mice cannot fully simulate human exercise and lifespan. The molecular mechanism, which involved many signaling pathways, needs to be conducted to collect better information about how exercise could involve in improving obesity or other related metabolic condition. However, the humanized mice model was important for preliminary studies before applied in human research.
Conclusion
In summary, the MI exercise was demonstrated to suppress the weight gain better than in the control group. Also, the adiponectin levels were highest in MI exercise, followed by HI, LI, and the control group. Based on our current results, MI exercise is most effective in reducing body weight and increasing serum adiponectin levels compared to other intensity exercise. These findings suggest that moderate-intensity swimming training could be considered a therapeutic option for preventing and treating obesity issues in murine models.
These findings support the notion that exercise intensity plays a crucial role in modulating adiponectin levels and metabolic outcomes in high-fructose-induced mice. Further exploration of the underlying mechanisms, application of these findings in clinical settings, and consideration of individual exercise prescriptions based on intensity levels could pave the way for more effective strategies in managing obesity and metabolic disorders.
However, it is crucial to acknowledge that these results, while promising, require further validation. The limitations of our study include the exclusive use of murine models, which do not fully replicate human physiology. Consequently, it is essential to conduct additional research to explore the molecular mechanisms involved and to confirm the applicability of these findings to human subjects. Further studies are needed to explore the applicability of these results in preventing and treating obesity in humans.
In conclusion, while our results provide a preliminary understanding of the benefits of moderate-intensity exercise, further research is needed to establish a strong scientific basis for its use in preventing and treating obesity in humans. Exploring the underlying mechanisms, applying these findings in clinical settings, and tailoring individual exercise prescriptions based on intensity levels will be crucial for developing more effective strategies to manage obesity and metabolic disorders.
References
1. Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med [Internet]. 2017;5(7):1-12. doi: https://doi.org/10.21037/atm.2017.03.107
2. Mamdouh H, Hussain HY, Ibrahim GM, Alawadi F, Hassanein M, et al. Prevalence and associated risk factors of overweight and obesity among adult population in Dubai: a population-based cross-sectional survey in Dubai, the United Arab Emirates. BMJ Open [Internet]. 2023;13(1):e062053. doi: https://doi.org/10.1136/bmjopen-2022-062053
3. Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res [Internet]. 2017;122:1-7. doi: https://doi.org/10.1016/j.phrs.2017.05.013
4. World Obesity Federation [Internet]. England & Wales: The Federation; c2022. World Obesity Atlas 2022; [about 3 screens]. Available from: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022
5. Basic Health Research (Riskesdas). National Report on Basic Health Research. Jakarta: Kemenkes RI. 2018. Available at: https://repository.badankebijakan.kemkes.go.id/id/eprint/3514/1/Laporan%20Riskesdas%202018%20Nasional.pdf
6. Ozcan Sinir G, Suna S, Inan S, Bagdas D, Tamer CE, Copur OU, et al. Effects of long-term consumption of high fructose corn syrup containing peach nectar on body weight gain in sprague dawley rats. Food Sci Technol (Campinas) [Internet]. 2017;37(2):337-43. doi: https://doi.org/10.1590/1678-457x.25416
7. Pereira RM, Botezelli JD, da Cruz Rodrigues KC, Mekari RA, Esper Cintra D, Pauli JR, et al. Fructose Consumption in the Development of Obesity and the Effects of Different Protocols of Physical Exercise on the Hepatic Metabolism. Nutrients [Internet]. 2017;9(4):1-21. doi: https://doi.org/10.3390/nu9040405
8. Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol [Internet]. 2016;8(2):93-100. doi: https://doi.org/10.1093/jmcb/mjw011
9. Vekic J, Zeljkovic A, Stefanovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metabolism [Internet]. 2019;92:71-81. doi: https://doi.org/10.1016/j.metabol.2018.11.005
10. Nguyen TMD. Adiponectin: Role in Physiology and Pathophysiology. Int J Prev Med [Internet]. 2020;11(1):136. doi: https://doi.org/10.4103/ijpvm.IJPVM_193_20
11. Aleidi S, Issa A, Bustanji H, Khalil M, Bustanji Y. Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm J [Internet]. 2015;23(3):250-6. doi: https://doi.org/10.1016/j.jsps.2014.11.011
12. Liu W, Zhou X, Li Y, Zhang S, Cai X, Zhang R, et al. Serum leptin, resistin, and adiponectin levels in obese and non-obese patients with newly diagnosed type 2 diabetes mellitus. A population-based study. Medicine (Baltimore) [Internet]. 2020;99(6):e19052. doi: https://doi.org/10.1097/MD.0000000000019052
13. Chakraborti CK. Role of adiponectin and some other factors linking type 2 diabetes mellitus and obesity. World J Diabetes [Internet]. 2015;6(15):1296-1308. doi: https://doi.org/10.4239/wjd.v6.i15.1296
14. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol [Internet]. 2021;320(3):C375-C391. doi: https://doi.org/10.1152/ajpcell.00379.2020
15. Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int [Internet]. 2014;2014:658913. doi: https://doi.org/10.1155/2014/658913
16. Arena R, Sagner M, Byrne NM, Williams AD, McNeil A, Street SJ, et al. Novel approaches for the promotion of physical activity and exercise for prevention and management of type 2 diabetes. Eur J Clin Nutr [Internet]. 2017;71(7):858-64. doi: https://doi.org/10.1038/ejcn.2017.53
17. Pranoto A, Cahyono MBA, Yakobus R, Izzatunnisa N, Ramadhan RN, Rejeki PS, et al. Long-Term Resistance-Endurance Combined Training Reduces Pro-Inflammatory Cytokines in Young Adult Females with Obesity. Sports (Basel) [Internet]. 2023;11(3):1-12. doi: https://doi.org/10.3390/sports11030054
18. Rejeki PS, Pranoto A, Rahmanto I, Izzatunnisa N, Yosika GF, Hernaningsih Y, et al. The Positive Effect of Four-Week Combined Aerobic-Resistance Training on Body Composition and Adipokine Levels in Obese Females. Sports (Basel) [Internet]. 2023;11(4):1-13. doi: https://doi.org/10.3390/sports11040090
19. Zhang Y, Xu J, Zhou D, Ye T, Zhou P, Liu Z, et al. Swimming exercise ameliorates insulin resistance and nonalcoholic fatty liver by negatively regulating PPARγ transcriptional network in mice fed high fat diet. Mol Med [Internet]. 2023;29(1):150. doi: https://doi.org/10.1186/s10020-023-00740-4
20. Krause MP, Milne KJ, Hawke TJ. Adiponectin-Consideration for its Role in Skeletal Muscle Health. Int J Mol Sci [Internet]. 2019;20(7):1-17. doi: https://doi.org/10.3390/ijms20071528
21. Zelikovich AS, Quattrocelli M, Salamone IM, Kuntz NL, McNally EM. Moderate exercise improves function and increases adiponectin in the mdx mouse model of muscular dystrophy. Sci Rep [Internet]. 2019;9(1):5770. doi: https://doi.org/10.1038/s41598-019-42203-z
22. Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev [Internet]. 2012;26(3):271-81. doi: https://doi.org/10.1101/gad.177857.111
23. Ge X, Chen C, Hui X, Wang Y, Lam KS, Xu A. Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes. J Biol Chem [Internet]. 2011;286(40):34533-41. doi: https://doi.org/10.1074/jbc.M111.248591
24. Lin Z, Tian H, Lam KS, Lin S, Hoo RCL, Konishi M, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17(5):779-89. doi: https://doi.org/10.1016/j.cmet.2013.04.005
25. Doulberis M, Papaefthymiou A, Polyzos SA, Katsinelos P, Grigoriadis N, Srivastava DS, et al. Rodent models of obesity. Minerva Endocrinol [Internet]. 2020;45(3):243-63. doi: https://doi.org/10.23736/S0391-1977.19.03058-X
26. Yu L, Fu M, Yang L, Sun H. Fasting Blood Glucose-Based Novel Predictors in Detecting Metastases and Predicting Prognosis for Patients with PNENs. J Pers Med [Internet]. 2024;14(7):1-15. doi: https://doi.org/10.3390/jpm14070760
27. Beck AP, Meyerholz DK. Evolving challenges to model human diseases for translational research. Cell Tissue Res [Internet]. 2020;380(2):305-11. doi: https://doi.org/10.1007/s00441-019-03134-3
28. Roberts FL, Markby GR. New Insights into Molecular Mechanisms Mediating Adaptation to Exercise; A Review Focusing on Mitochondrial Biogenesis, Mitochondrial Function, Mitophagy and Autophagy. Cells [Internet]. 2021;10(10):1-29. doi: https://doi.org/10.3390/cells10102639
29. Yuliastrid D, Kusnanik NW, Purwanto B, Noordia A, Purwoto SP, Pranoto A. Single bout of a long-duration running treadmill increases myoglobin but not haemoglobin and interleukin 6 levels in mice (Mus musculus). Comp Exerc Physiol [Internet]. 2023;19(4):353-9. doi: https://doi.org/10.1163/17552559-20220075
30. Prasetya RE, Umijati S, Rejeki PS. Effect of Moderate Intensity Exercise on Body Weight and Blood Estrogen Level Ovariectomized Mice. Majalah Kedokteran Bandung [Internet]. 2018;50(3):147-51. doi: https://doi.org/10.15395/mkb.v50n3.1368
31. Sari DR, Ramadhan RN, Agustin D, Munir M, Izzatunnisa N, Susanto J, et al. The Effect of Exercise Intensity on Anthropometric Parameters and Renal Damage in High Fructose-Induced Mice. Retos [Internet]. 2024;51:1194-209. doi: https://doi.org/10.47197/retos.v51.101189
32. Wigati KW, Bintari MP, Rejeki PS, Wungu CDK, Pranoto A, Ramadhan RN, et al. The effect of 4 week-long swimming exercise intervention on increased serotonin levels in male mice (Mus musculus). Comp Exerc Physiol [Internet]. 2023;19(4):361-70. doi: https://doi.org/10.1163/17552559-20230005
33. Riahi F, Riyahi S. Effect of Moderate Swimming Exercise on Weight Gain in High Fat Diet Rats. Ann Mil Health Sci Res [Internet]. 2016;14(1):e13819. Available from: https://brieflands.com/articles/amhsr-13819
34. Acikel Elmas M, Cakıcı SE, Dur IR, Kozluca I, Arınc M, Binbuga B, et al. Protective effects of exercise on heart and aorta in high-fat diet-induced obese rats. Tissue Cell [Internet]. 2019;57:57-65. doi: https://doi.org/10.1016/j.tice.2019.01.005
35. Kolieb E, Maher SA, Shalaby MN, Alsuhaibani AM, Alharthi A, Hassan WA, et al. Vitamin D and Swimming Exercise Prevent Obesity in Rats under a High-Fat Diet via Targeting FATP4 and TLR4 in the Liver and Adipose Tissue. Int J Environ Res Public Health [Internet]. 2022;19(21):1-22. doi: https://doi.org/10.3390/ijerph192113740
36. Antoni MF, Rejeki PS, Sulistiawati, Pranoto A, Wigati KW, Sari GM, et al. Effect of nocturnal and diurnal moderate-intensity swimming exercise on increasing irisin level of female mice (Mus musculus). CMUJ Nat Sci [Internet]. 2022;21(2):e2022033. Available from: https://repository.unair.ac.id/116850/
37. Chen YM, Lian CF, Sun QW, Wang TT, Liu YY, Ye J, et al. Ramulus Mori (Sangzhi) Alkaloids Alleviate High-Fat Diet-Induced Obesity and Nonalcoholic Fatty Liver Disease in Mice. Antioxidants (Basel) [Internet]. 2022;11(5):1-19. doi: https://doi.org/10.3390/antiox11050905
38. Guo S, Huang Y, Zhang Y, Huang H, Hong S, Liu T. Impacts of exercise interventions on different diseases and organ functions in mice. J Sport Health Sci [Internet]. 2020;9(1):53-73. doi: https://doi.org/10.1016/j.jshs.2019.07.004
39. Rahayu FK, Dwiningsih SR, Sa'adi A, Herawati L. Effects of different intensities of exercise on folliculogenesis in mice: Which is better?. Clin Exp Reprod Med [Internet]. 2021;48(1):43-9. doi: https://doi.org/10.5653/cerm.2020.03937
40. Rezaie P, Mazidi M, Nematy M. Ghrelin, food intake, and botanical extracts: A Review. Avicenna J Phytomed [Internet]. 2015;5(4):271-81. doi: https://doi.org/10.22038/ajp.2015.4196
41. Sholikhah AM, Ridwan M. Swimming training on moderate intensity significantly reduces total cholesterol and bodyweight on hypercholesterolemic rat model. Jurnal Keolahragaan [Internet]. 2021;9(1):51-8. doi: https://doi.org/10.21831/jk.v9i1.33362
42. Alfin R, Busjra B, Azzam R. [The Effect of Ramadan Fasting on Blood Sugar Levels in Type II Diabetes Mellitus Patients]. Journal of Telenursing (JOTING) [Internet]. 2019;1(1):191-204. doi: https://doi.org/https://doi.org/10.31539/joting.v1i1.499
43. Nakrani MN, Wineland RH, Anjum F. Physiology, Glucose Metabolism. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560599/
44. Geng L, Liao B, Jin L, Huang Z, Triggle C, Ding H, et al. Exercise Alleviates Obesity-Induced Metabolic Dysfunction via Enhancing FGF21 Sensitivity in Adipose Tissues. Cell Rep [Internet]. 2019;26(10):2738-52.e4. doi: https://doi.org/10.1016/j.celrep.2019.02.014
45. Jortay J, Senou M, Abou-Samra M, Noel L, Robert A, Many MC, et al. Adiponectin and skeletal muscle: pathophysiological implications in metabolic stress. Am J Pathol [Internet]. 2012;181(1):245-56. doi: https://doi.org/10.1016/j.ajpath.2012.03.035
46. Martinez-Huenchullan SF, Maharjan BR, Williams PF, Tam CS, Mclennan SV, Twigg SM. Differential metabolic effects of constant moderate versus high intensity interval training in high-fat fed mice: possible role of muscle adiponectin. Physiol Rep [Internet]. 2018;6(4):e13599. doi: https://doi.org/10.14814/phy2.13599
47. Xie Y, Li Z, Wang Y, Xue X, Ma W, Zhang Y, Wang J, et al. Effects of moderate- versus high- intensity swimming training on inflammatory and CD4+ T cell subset profiles in experimental autoimmune encephalomyelitis mice. J Neuroimmunol [Internet]. 2019;328:60-7. doi: https://doi.org/10.1016/j.jneuroim.2018.12.005
48. Lu Y, Bu FQ, Wang F, Liu L, Zhang S, Wang G, et al. Recent advances on the molecular mechanisms of exercise-induced improvements of cognitive dysfunction. Transl Neurodegener [Internet]. 2023;12(1):9. doi: https://doi.org/10.1186/s40035-023-00341-5
49. Achari AE, Jain SK. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int J Mol Sci [Internet]. 2017;18(6):1-17. doi: https://doi.org/10.3390/ijms18061321