Pilot Study of the Effectiveness of Repetitive Transcranial Magnetic Stimulation on Pain and Quality of Life in Patients with Chemotherapy-Induced Peripheral Neuropathic Pain
Estudio piloto de la eficacia de la estimulación magnética transcraneal repetitiva sobre el dolor y la calidad de vida en pacientes con dolor neuropático periférico inducido por quimioterapia
Catalina Lopera-Muñeton , Silvia Betancur-Bedoya, Isabel Angel, María Guadalupe Vásquez-Montoya, Sebastian Grajales-Toro, Dionis Vallejo
Abstract
Background: Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive technique that acts on the activity of the cerebral cortex employing electrical currents.
Aim: The objective of this project is to evaluate the effectiveness of rTMS on pain and quality of life in patients with chemotherapy-induced peripheral neuropathic pain.
Method: Ten patients with chemotherapy-induced peripheral neuropathic pain received 20 sessions of rTMS, consisting of 15 minutes of treatment repeated 5 times per week for four weeks (10 Hz, 20s, 30 trains with 81% intensity). Patients were evaluated using the Brief pain inventory (BPI) and the Functional Assessment of Cancer Therapy and neurotoxicity (FACT-GOG-NTX 13).
Results: There were significant differences in BPI mean severity, interference score and FACT-GOG-NTX 13 (p<0,05).
Conclusion: The pilot study results suggest that rTMS is potentially beneficial for the treatment of chemotherapy-induced peripheral neuropathy. rTMS over the M1 had an important reduction in pain severity, interference with daily activities, and quality of life scores. However, results should be taken with caution due to the small sample size, absence of a control group and short period of follow-up.
Keywords
Repetitive transcranial magnetic stimulation; chemotherapy-induced peripheral neuropathic pain; cancer; quality of life; physiotherapy.
Resumen
Antecedentes: La estimulación magnética transcraneal repetitiva (EMTr) es una técnica no invasiva que actúa sobre la actividad de la corteza cerebral, empleando corrientes eléctricas.
Objetivo: El objetivo de este proyecto es evaluar la eficacia de la rTMS sobre el dolor y la calidad de vida en pacientes con dolor neuropático periférico inducido por quimioterapia.
Métodos: Diez pacientes con dolor neuropático periférico inducido por quimioterapia recibieron 20 sesiones de EMTr que consistieron en un tratamiento de 15 minutos repetido 5 veces por semana durante cuatro semanas (10 Hz, 20 s, 30 trenes con 81 % de intensidad). Los pacientes fueron evaluados mediante el Inventario Breve de Dolor (BPI) y la Evaluación Funcional de la Terapia del Cáncer y la neurotoxicidad (FACT-GOG-NTX 13).
Resultados: Hubo diferencias significativas en la severidad media del dolor del BPI, la puntuación de interferencia y el FACT-GOG-NTX 13 (p<0,05).
Conclusión: Los resultados del estudio piloto sugieren que la rTMS es potencialmente beneficiosa para el tratamiento de la neuropatía periférica inducida por la quimioterapia. La rTMS sobre M1 tuvo una reducción importante de la severidad del dolor, la interferencia con las actividades diarias y las puntuaciones de calidad de vida. Sin embargo, los resultados deben tomarse con cautela debido al pequeño tamaño de la muestra, la ausencia de un grupo de control y el corto período de seguimiento.
Palabras clave
Estimulación magnética transcraneal repetitiva; dolor neuropático periférico inducido por quimioterapia; cáncer; calidad de vida; fisioterapia.
Introduction
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive and painless technique that acts on the activity of the cerebral cortex employing electrical currents [1]. It is used to treat diseases and symptoms associated with neuropathic pain in cases such as orofacial pain [2], central neuropathic pain [3], diabetic neuropathic pain [4], and even in neuropathies after cancer [5]. According to the American Society of Clinical Oncology (ASCO), at least 3.5 million people around the world suffer or feel the pain associated with cancer [6]. The prevalence of pain due to neurological lesions in patients with advanced cancer can reach 40% [7]. In Latin America, the specific incidence of cancer neuropathic pain is unknown. However, 2% of the general population suffers from neuropathic pain (NP), a figure that is most likely underestimated [8]. Furthermore, considering existing clinical guidelines for pain management such as those of the National Comprehensive Cancer Network (NCCN), pain can be clinically classified into three main subtypes according to neurophysiology and neuroanatomy: somatic, visceral, and, the one that concerns this research, neuropathic cancer pain. This type of pain is caused by multiple factors, such as surgical procedures, tumor nerve compression, and chemotherapy [9]. Neuropathic pain (NP) is generally described as discharges of burning or stinging sensations, prickling, numbness or paresthesia [10]. According to the International Association for the Study of Pain (IASP), NP is caused by a primary lesion or transitory dysfunction of the somatosensory system, which causes several alterations that considerably deteriorate the quality of life of this population [11].
Chemotherapy-induced peripheral neuropathy (CIPN) is characterized by a subacute onset, with a progressive course, appearing after several cycles of treatment and usually disappearing within approximately 48 hours of application. About 30% of patients will continue to have CIPN for a year or even longer after chemotherapy ends. Each antitumor drug causes a different type of alteration in the genome. On the one hand, platinum (cisplatin, oxaliplatin) produced a mechanism of damage in mitochondrial and nuclear DNA. Vinca alkaloids, such as vincristine, and the paclitaxel family (Docetaxel, Cabalitaxel), destabilize the microtubules of the cytoskeleton of cells. Another group is Bortezomib (Carfilzomib, Ixazomib), which are inhibitor of the kinase activity of the 26S proteasome. And the last group of drugs has antiangiogenic activity, as the antineoplastic drugs (thalidomide, Lenalidomina, Pomalidomina) also affect sensory nerves [12].
For several years, the management of neuropathic pain due to cancer and non-oncological pathologies was exclusively limited to medications, including antidepressants, anticonvulsants, and opioids [13]. However, according to current estimates, about 5.7 million people in Latin America and the Caribbean have limited access to analgesics. In the last years Colombia, Argentina, Chile, Guatemala, and Honduras have described low access levels to these medications, indicating that pain management is close to deficient in large part of the Latin American population [14]. In addition, the guidelines established in the topic, include neuromodulation as a third-line treatment for neuropathic pain [15,16].
Hence, repetitive transcranial magnetic stimulation is a modality recently incorporated as a therapeutic option for NP. rTMS is a non-invasive brain stimulation technology that employs a magnetic field, which intends to normalize brain functions associated with pain-processing in the primary motor cortex area (M1) [17]. rTMS induces neuroplasticity which translates into pain modulation [18].
There is evidence regarding the effectiveness of rTMS for neuropathic pain [19-21]. However, there is poor information specifically for oncological population [5,22].
Therefore, this project aims to evaluate the effectiveness of rTMS on pain and quality of life in patients with chemotherapy-induced peripheral neuropathic pain.
Methods
Study design
A descriptive correlational study was carried out in ten patients with CIPN after rTMS application. Ethical approval was given by Fundación Universitaria María Cano # 013008028-2021-311. The study protocol was registered in clinical trials.gov (https://clinicaltrials.gov/) ID NCT05480410. The protocol was structured according to the National Institute of Health, the statements described by the clinicaltrial database, and the SPIRIT declaration, which includes detailed study description and design, intervention, outcome measures, individual participant data and results sharing statement [23,24]. All participants provided written informed consent prior to participation in the study. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines [25].
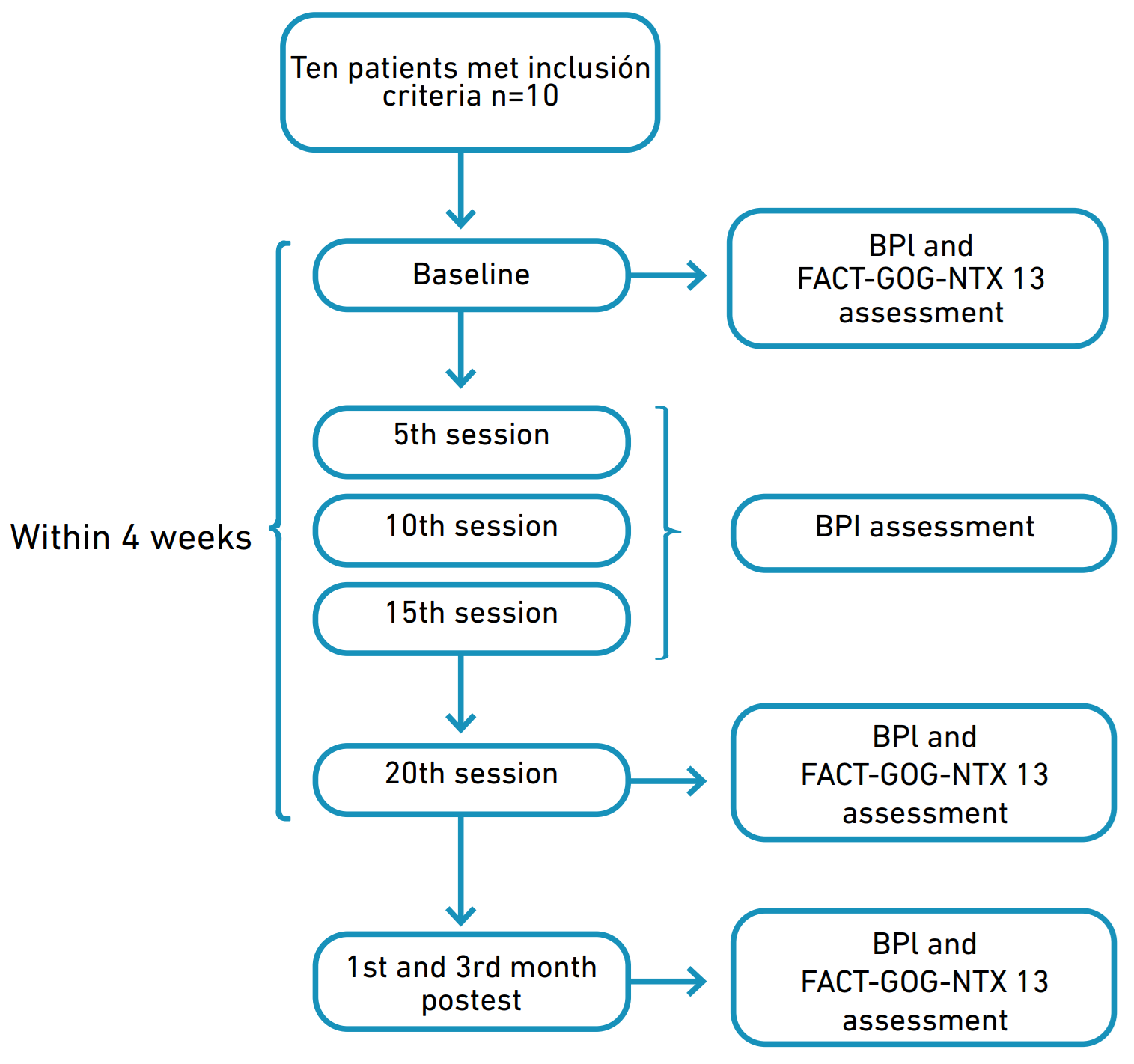
This study was conducted at Neuroclínica Colombia from June to December 2022. Patients were recruited from the research center in Fundación Universitaria María Cano. The patients met the following inclusion criteria: female or males aged over 18 and under 75 years, any stage of cancer, chemotherapeutic treatment consisting of taxanes or oxaliplatin, neuropathic pain with a minimum grade 2 severity based on the National Cancer Institute Common Terminology Criteria for Adverse Events scale (NCI-CTCAE, version 5.0) [26], and a mean 2 score or above in a visual analogue scale (VAS) of pain. We excluded patients with any psychiatric disorder including major depression; history of seizure, epilepsy, stroke, and intracranial metallic devices. Patients agreed to avoid any extra use of medication or to make any changes to their pharmacological plan during the trial. Figure 1 illustrates a flow chart of the organizational structure of the approved study.
Figure 1. Flowchart of the organizational structure of the study.
Evaluation
All the patients had CIPN diagnosed by a neurologist and NCI-CTCAE scale. Participants were evaluated by a physiotherapist aiming to assess baseline pain-related functional impairment using the brief pain inventory (BPI) and quality of life by the Functional Assessment of Cancer Therapy (FACT-GOG-NTX 13). Health related quality of life (HRQoL) was measured based on the Ferrell et al. model, which describes four general domains: Physical, Psychological, Social, and Emotional [27]. Assessment was made using the questionnaire from the FACIT measurement system, which has designed more than 700 instruments to quantify health-related quality of life for people with cancer
Determination of resting motor threshold
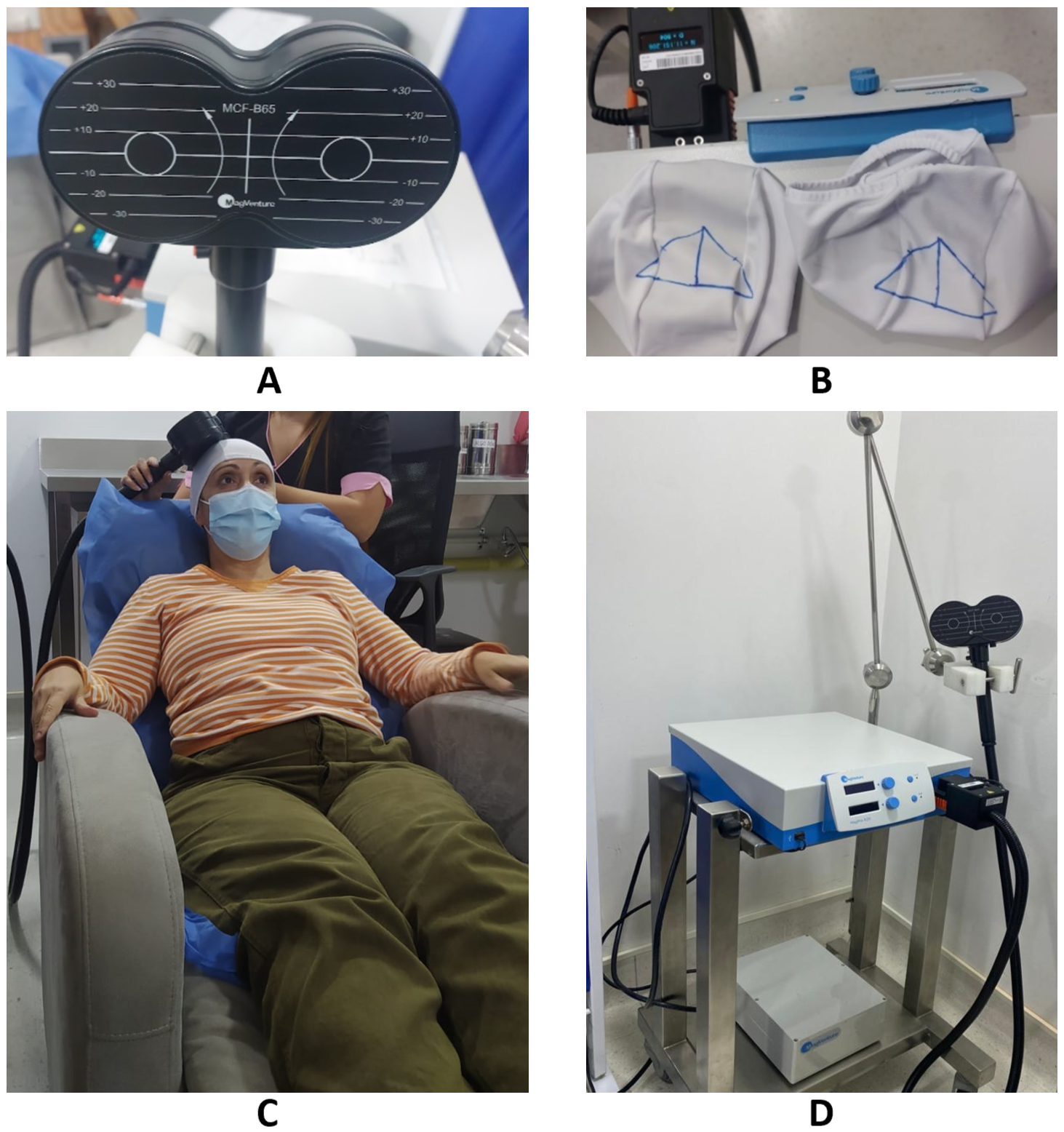
The target stimulation was the primary motor cortex in its subdivision representing the hand. For this process, a basic trained operator identified the ‘omega-shape’ sulcus defining the Rolandic sulcus. Other authors have reported this specific hot spot target including the expert consensus established by a group of European experts. They described a Level A recommendation for the use of M1 as stimulation site for analgesic effect [5,20,29-32]. Then, the accurate position of the target was adjusted according to the amplitude of the motor response on the contralateral hand, which a basic rTMS-trained physiotherapist confirmed. This target has been shown in different investigations to have greater specificity and effectiveness in the treatment of neuropathic pain [33,34]. Once the target was identified, it was marked on a cap for the following sessions (Figure 2). A physiotherapist was present weekly for assessment and to supervise patients’ responses to treatment.
Figura 2. Figure-8 coil (A). Target marked on cap (B) rTMS therapy delivered (C) Image of the MagPro R20 magnetic stimulator (D).
Intervention
The therapy was delivered with a Magpro R20 stimulator (Magventure Tonika Elektronic, Farum, Denmark) through a figure eight-coil. Stimulation parameters were based on guideline recommendations [20]. They consisted of 30 consecutive trains of 100 stimulations delivered at 10 Hz, at 81% of motor threshold, separated by inter-train intervals of 20 seconds, with a total of 3000 stimulations during session. The protocol consisted of 15-minute sessions repeated 5 times per week for four weeks (20 sessions). These parameters were selected based on previous research that described a higher percentage of pain relief in the long term after 15-20 sessions on average [35] and delivered in a session of 15 minutes [30]. The occurrence of adverse events was noted throughout the study period.
Follow-up measures
For follow up measures, a physiotherapist assessed pain on the 5th, 10th, 15th and 20th session after rTMS intervention and 1st and 3rd month post-test; quality of life was assessed on the 20th session after rTMS intervention and 1st and 3rd month post-test. Information was collected through the Brief Pain Inventory and the Functional Assessment of Cancer Therapy. BPI allows patients to rate the severity of their pain and the degree to which their pain interferes with typical dimensions of feeling and function. It includes a 0-10 numerical rating scale (NRS) to measure four pain severity items: worst, least, average, and actual pain. All these items are included in a mean severity score, which classified pain as follows: mild pain (score 1-4), moderate pain (score 5-6), and severe pain (score 7-10). In addition, the interference score measures how pain affects some daily activities, including general activity, mood, walking, work, relations with others, sleep, and enjoyment of life. BPI pain interference is typically scored as the mean of the seven interference items (0-10), where 0 indicates that the pain does not interfere and ten completely interferes. The BPI was selected as it is a tool designed to assess pain related to cancer [36,37] and was found to demonstrate good internal stability (Cronbach’s alpha >0.70), as well as test-retest reliability [38].
The Functional Assessment of cancer Therapy/Gynecologic Oncology Group Neurotoxicity (FACT-GOG-NTX 13) was used to assess quality of life in neuropathy. It includes a 27-item general questionnaire designed to measure four health-related quality of life domains in cancer patients: physical, social, emotional, and functional well-being. In addition, it includes a 13-item neurotoxicity (NTX-13) module added to the core questionnaire. Each item is scored on a 5-point scale (0 = not at all, 4 = very much), and all subscale items are summed to a total (FACT G/Total score), where a higher score indicates a better quality of life (QOL). This instrument has evidenced satisfactory reliability, validity, sensitivity to change, and responsiveness to evaluate neuropathic pain [39]. In addition, previous authors have evaluated the psychometric properties of the FACT Spanish version with Colombian patients [40].
Data analysis
A univariate descriptive study was carried out using measures of frequency and central tendency, depending on the nature of the variable. Normality tests were made using Shapiro wilks statistic measure, finding non parametric variables. The difference of two variables of related samples was carried out using the Wilcoxon test. Finally, the analysis of variance (ANOVA) test was used to determine the effect of two nominal predictor variables on a continuous outcome variable.
Results
Ten patients were included in this pilot study. The mean age of the studied patients was 52.4± 12.6 years, (1 man and 9 women). Nine patients had breast cancer and one multiple myeloma. Baseline characteristics are described in Table 1.
Table 1. Baseline characteristics of participants.
| Parameters | Volunteers | % | |
|---|---|---|---|
| Age (Years) | Mean± (SD) | 52.4± 12.6 | n=10 |
| Socioeconomic level | 1 | 0% | |
| 2 | 20% | ||
| 3 | 60% | ||
| 4 | 10% | ||
| 5 | 10% | ||
| Educative level | Elementary school | 10% | |
| High school | 20% | ||
| Technical institution | 30% | ||
| University | 30% | ||
| Postgraduate degree | 10% | ||
| Cancer Stage | I | 20% | |
| II | 60% | ||
| III | 20% | ||
| Time after Chemotherapy | 1-3 Months | 10% | |
| 4-7 Months | 50% | ||
| 8-12 Months | 10% | ||
| 13 Months or more | 30% | ||
| Body Mass index (BMI) | Mean± (SD) | 30.1± 5.5 | n=10 |
Nota. SD: Standard deviation.
Scores on all the rating scales changed over time. For the brief pain inventory, the mean-SD pain severity score at baseline was 5,53±1,27 classified as moderate pain. These rates showed significant reduction at 10th, 15th, and 20th session, including the 1st month post-test (p<0,005). In addition, at initial evaluation participants reported a mean-SD interference score of 5,75±1,58, there was a reduction at session 5th, 10th, 15th, and 20th, reporting a mean-SD of 3,11±2,35, 2,35±1,75, 1,25±1,15, and 0,73±1,03, respectively, with a significant difference compared to baseline (p<0,05). These significant differences were maintained at 1st and 3rd month post-test with a mean-SD score of 2,01±1,59 and 3,30±2,27, respectively. Mean BPI scores are shown in Table 2.
Table 2. Mean scores of BPI at baseline, 5th, 10th, 20th session, 1st month and 3rd month post-test.
| Assessment | BPI Mean pain severity score Range (0-10) | BPI Mean Interference score Range (0-10) | ||
|---|---|---|---|---|
| Mean-SD | p value | Mean-SD | p value | |
| Baseline | 5,53±1,27 | 5,75±1,58 | ||
| 5th session | 4,25±2,64 | 0,113 | 3,11±2,35 | 0,020* |
| 10th session | 2,50±2,26 | 0,005* | 2,35±1,75 | 0,000* |
| 15th session | 1,72±1,73 | 0,000* | 1,25±1,15 | 0,000* |
| 20th session | 1,60±1,47 | 0,000* | 0,73±1,03 | 0,000* |
| 1st month post-test | 1,97±1,77 | 0,000* | 2,01±1,59 | 0,000* |
| 3rd month post-test | 3,48±2,65 | 0,057 | 3,30±2,27 | 0,018* |
Nota. BPI: Brief Pain inventory; SD: Standard deviation; * Statistical significance.
The patient’s FACT G mean-SD total score increased from 68,90±7,62 points at baseline to 91,00±9,23 at 20th session (p=0,001), which was the highest score. FACT G mean-SD total score decreased at 1st and 3rd month post-test. However, scores maintain a significant difference compared to baseline (p<0,05), which implies a better quality of life rate after treatment and at follow up. A similar behavior was observed for the neurotoxicity scale with a baseline mean-SD score of 27,60±8,69 and a significant increase at the end of treatment with a mean-SD score of 38,10±7,51 (p=0,012). Although mean rate decreased at 1 month follow up, there still was a significant difference compared to baseline 36,20±7,88 (p= 0,011). Mean FACT-GOG NTX scores are shown in Table 3.
Table 3. Mean scores of FACT-GOG NTX at baseline, 20th session, 1st month and 3rd month post-test.
| Assessment | Trial Outcome Index (TOI) | FACT G- total score | Neurotoxicity scale | |||
|---|---|---|---|---|---|---|
| Range (0-100) | Range (0-108) | Range (0-52) | ||||
| Mean-SD | p value | Mean-SD | p value | Mean-SD | p value | |
| Baseline | 60,50±10,59 | 68,90±7,62 | 27,60±8,69 | |||
| 20th session | 85,20±15,08 | 0,001* | 91,00±9,23 | 0,001* | 38,10±7,51 | 0,012* |
| 1st month post-test | 80,10±14,30 | 0,001* | 85,10±15,08 | 0,003* | 36,20±7,88 | 0,011* |
| 3rd month post-test | 75,70±18,57 | 0,062 | 79,50±16,90 | 0,007* | 35,10±10,21 | 0,100 |
Nota. FACT GOG- NTX: Functional Assessment of cancer Therapy/Gynecologic Oncology Group- Neurotoxicity; SD: Standard deviation; * Statistical significance.
The Muchli sphericity test from the ANOVA analysis was performed to study the effect of time on the BPI severity score; the results described a statistical significance (p=0.001) and a partial ETA squared of 45%. This indicates that the remaining 55% of the evaluated population improved pain levels specifically due to rTMS intervention.
Patients reported no significant secondary effects, including mild headache and drowsiness for the first session. Those participants were referred to the neurologist who reported symptoms as transitory effects without any potential risk to the participant's health.
Discussion
The goal of this pilot study was to investigate the effectiveness of rTMS treatment on CIPN. These results suggest that rTMS at 10 Hz given every day for 20 sessions has the potential effect to reduce ratings of pain and improve quality of life in this population. There was a significant reduction in BPI mean pain severity and mean pain interference score at 10th, 15th, and 20th session and 1st month post-test (p<0,05). In addition, the mean interference score showed a statistically significant reduction at 3rd month follow up. Regarding quality of life, the TOI and Neurotoxicity scales reported a significant increase at the end of treatment and 1st month post-test (p< 0,05). The FACT G total score increased at 20th session and 1st and 3rd month post-test (p<0,005). These results support the statement defined by the evidence-based guidelines on rTMS, which described that the analgesic effects of the treatment are associated with an improvement in quality of life scores [20]. In addition, even though 70% of CIPN patients improve spontaneously during the 6 months [41], according to the present results, 55% of the patients included improved pain levels specifically due to rTMS intervention, regardless of the time elapsed from the end of treatment.
Hence, rTMS is a non-invasive procedure that has been recognized as an effective treatment for intractable neuropathic pain from different origins [17,42-44]. The literature describes that rTMS induces plastic changes in the central nervous system by increasing blood flow, changing the resting membrane potential, modulating neuroinflammation, and releasing endogenous opioids [18]. Therefore, this technology has been suggested as a safe alternative treatment option that relieves NP by modulating cortical excitability [21].
There is scarce evidence related specifically to rTMS in oncologic population. Thus, Khedr et al. [5] included thirty-four cancer patients who received real rTMS (20 Hz, 10 s, 10 trains with 80% intensity) or sham rTMS daily for 10 consecutive days. Patients were assessed using a verbal descriptor scale (VDS), a visual analogue scale (VAS), the Leeds assessment of neuropathic symptoms and signs (LANSS), and the Hamilton rating scale for depression (HAM-D) at baseline after the first, fifth, and tenth treatment sessions. Follow up was assessed 15 days and 1 month after treatment. They reported a higher reduction of VAS in the real group after the 10th session (p = 0,001). Further, this effect persisted at 15 days of follow up. There was a significant reduction of the HAM-D in the real group after the 10th session (p=0,002). These effects persisted at 1 month follow up (p= 0,038). Similarly, our results showed significant reduction in mean pain severity, interference, and quality of life, which persisted for all the scores after 1 month follow up. HRQOL is usually assessed via multiple indicators of self-perceived health status, physical and emotional functioning [45]. Hence, in our study quality of life was evaluated by the FACT-GOG-NTX 13 instrument, which is compound by 4 subscales (physical, functional, emotional, and social domain) according to the Ferrell et al. model [27]. These results suggest the complexity of neuropathic pain, which involves not only physical comorbidities, but also psychological alterations such as depression and anxiety. These elements have a negative impact on quality of life and its multiple dimensions, generating disabilities and social restrictions [46]. Therefore, there is a need to promote a comprehensive intervention for neuropathic cancer pain as they are a vulnerable and undertreated population [47].
Goto et al. [22] included a total of 11 female patients with chemotherapy-induced peripheral neuropathy, who received four types of rTMS for two months. Pain was assessed using the VAS Visual Analogue Scale (P-VAS) and the Japanese version of the McGill Pain Questionnaire 2 (SF-MPQ2). Dysesthesia was evaluated using D-VAS. Assessment was performed at baseline, before stimulation, immediately after stimulation, and one hour after stimulation. Dysesthesia and pain scores significantly decreased after treatment, this suggests that rTMS may modulate altered nerve conduction in cancer patients. Therefore, evidence suggests that rTMS could be an alternative treatment strategy for this late effect, which has a prevalence up to 85% of cases [48].
Limitations and recommendations
Limitations in the interpretation of our results include the small sample size of this pilot study and the lack of a sham stimulation group. Further and larger studies on the effects of rTMS on neuropathic cancer pain are needed in order to lead to advancements in the clinical application and effectiveness of the technique. Future research with longer follow up could support the inclusion of rTMS as a therapeutic option for comprehensive oncology rehabilitation programs, which could improve pain modulation and positively impact quality of life. Corresponding with our results some of the scores did not show a statistically significant difference at 3rd month follow up, which suggests the possible need of some extra sessions to maintain therapeutical effect through time. In addition, according to the group of European experts on the therapeutic use of rTMS, the evidence suggests that the analgesic effect is favored by longer session duration and serial treatment with a greater number of sessions [20].
Conclusion
The pilot study results suggest that rTMS is potentially beneficial for the treatment of chemotherapy-induced peripheral neuropathy. However, results should be taken with caution due to small sample size, absence of control group, and short period of follow up. Twenty sessions of rTMS over the M1 had a beneficial reduction of pain severity, interference with daily activities, and quality of life scores. The maximum effect was reach at the end of treatment and 1st month follow up, with significant difference for all the elements assessed compare to baseline. Given the limitations of currently available medications and its secondary effects, the results suggest that rTMS is a favorable and non-invasive therapeutic option for cancer patients.
References
1. Hong P, Liu Y, Wan Y, Xiong H, Xu Y. Transcranial direct current stimulation for migraine: a systematic review and meta‐analysis of randomized controlled trials. CNS Neurosci Ther [Internet]. 2022;28(7): 992-8. doi: https://doi.org/10.1111/cns.13843
2. Lindholm P, Lamusuo S, Taiminen T, Pesonen U, Lahti A, Virtanen A, et al. Right secondary somatosensory cortex-a promising novel target for the treatment of drug-resistant neuropathic orofacial pain with repetitive transcranial magnetic stimulation. Pain [Internet]. 2015;156(7):1276-83. doi: https://doi.org/10.1097/j.pain.0000000000000175
3. Quesada C, Pommier B, Fauchon C, Bradley C, Créac'h C, Murat M, et al. New procedure of high-frequency repetitive transcranial magnetic stimulation for central neuropathic pain: a placebo-controlled randomized crossover study. Pain [Internet]. 2020;161(4):718-28. doi: https://doi.org/10.1097/j.pain.0000000000001760
4. Abdelkader AA, El Gohary AM, Mourad HS, El Salmawy DA. Repetitive TMS in treatment of resistant diabetic neuropathic pain. Egypt J Neurol Psychiatr Neurosurg [Internet]. 2019;55(1). doi: https://doi.org/10.1186/s41983-019-0075-x
5. Khedr EM, Kotb HI, Mostafa MG, Mohamad MF, Amr SA, Ahmed MA, et al. Repetitive transcranial magnetic stimulation in neuropathic pain secondary to malignancy: a randomized clinical trial. Eur J Pain [Internet]. 2015;19(4):519-27. doi: https://doi.org/10.1002/ejp.576
6. Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol [Internet]. 2014;32(18):1941-67. doi: https://doi.org/10.1200/JCO.2013.54.0914
7. Shkodra M, Caraceni A. Treatment of Neuropathic Pain Directly Due to Cancer: An Update. Cancers [Internet]. 2022;14(8). doi: https://doi.org/10.3390/cancers14081992
8. Correa-Illanes G. Dolor neuropático, clasificación y estrategias de manejo para médicos generales. Revista Médica Clínica Las Condes [Internet]. 2014;25(2):189-99. doi: https://doi.org/10.1016/S0716-8640(14)70030-6
9. Yoon SY, Oh J. Neuropathic cancer pain: prevalence, pathophysiology, and management. Korean J Intern Med [Internet]. 2018;33(6):1058-69. doi: https://doi.org/10.3904/kjim.2018.162
10. Swarm RA, Abernethy AP, Anghelescu DL, Benedetti C, Buga S, Cleeland C, et al. Adult Cancer Pain. J Natl Compr Canc Netw [Internet]. 2013;11(8):992-1022. doi: https://doi.org/10.6004/jnccn.2013.0119
11. Aydede M. Defending the IASP Definition of Pain. Monist [Internet]. 2017;100(4):439-64. doi: https://doi.org/10.1093/monist/onx021
12. Varona Sáez de Ibarra R. Mecanismos implicados en el desarrollo de Neuropatía periférica inducida por quimioterapia [undergraduate thesis]. Santander: Universidad de Cantabria; 2020. 45 p. Available from: https://repositorio.unican.es/xmlui/handle/10902/19486
13. Cruccu G, Truini A. A review of Neuropathic Pain: From Guidelines to Clinical Practice. Pain Ther [Internet]. 2017;6(Suppl 1):35-42. doi: https://doi.org/10.1007/s40122-017-0087-0
14. Díaz Juvier YL, Hernández Ortega Y, Hernández Rodríguez LA, Cuevas Pérez OL, Fernández Ruiz DR. Tratamiento del dolor en el paciente oncológico. MediSur [Internet]. 2019;17(4):552-61. Available from: https://pesquisa.bvsalud.org/portal/resource/pt/biblio-1091206
15. Moisset X, Bouhassira D, Avez Conturier J, Alchaar H, Conradi S, Delmotte MH, et al. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Rev Neurol (Paris) [Internet]. 2020;176(5):325-52. doi: https://doi.org/10.1016/j.neurol.2020.01.361
16. Thouaye M, Yalcin I. Neuropathic pain: From actual pharmacological treatments to new therapeutic horizons. Pharmacology & Therapeutics [Internet]. 2023;251:108546. doi: https://doi.org/10.1016/j.pharmthera.2023.108546
17. Hosomi K, Sugiyama K, Nakamura Y, Shimokawa T, Oshino S, Goto Y, et al. A randomized controlled trial of 5 daily sessions and continuous trial of 4 weekly sessions of repetitive transcranial magnetic stimulation for neuropathic pain. Pain [Internet]. 2020;161(2):351-60. doi: https://doi.org/10.1097/j.pain.0000000000001712
18. Ströher Toledo R, Stein DJ, Stefani Sanches PR, da Silva LS, Richardt Medeiros H, Fregni F, et al. rTMS induces analgesia and modulates neuroinflammation and neuroplasticity in neuropathic pain model rats. Brain Research [Internet]. 2021;1762:147427. doi: https://doi.org/10.1016/j.brainres.2021.147427
19. Lefaucheur JP, Ayache SS, Sorel M, Farhat WH, Zouari HG, Ciampi de Andrade D, et al. Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: influence of theta burst stimulation priming. Eur J Pain [Internet]. 2012;16(10):1403-13. doi: https://doi.org/10.1002/j.1532-2149.2012.00150.x
20. Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin Neurophysiol [Internet]. 2020;131(2): 474-528. doi: https://doi.org/10.1016/j.clinph.2019.11.002
21. Yang S, Chang MC. Effect of Repetitive Transcranial Magnetic Stimulation on Pain Management: A Systematic Narrative Review. Front Neurol [Internet]. 2020;11:1-19. doi: https://doi.org/10.3389/fneur.2020.00114
22. Goto Y, Hosomi K, Shimokawa T, Shimizu T, Yoshino K, Kim SJ, et al. Pilot study of repetitive transcranial magnetic stimulation in patients with chemotherapy-induced peripheral neuropathy. J Clin Neurosci [Internet]. 2020;73:101-7. doi: https://doi.org/10.1016/j.jocn.2020.01.020
23. Clinical Trials Registration and Results Information Submission. 42 CFR Part 11. 2016. Federal Register Vol. 81, No. 183 (Sep 21, 2016). Available from: https://www.federalregister.gov/documents/2016/09/21/2016-22129/clinical-trials-registration-and-results-information-submission
24. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krle A-Jerić K, et al. Declaración SPIRIT 2013: definición de los elementos estándares del protocolo de un ensayo clínico. Rev Panam Salud Publica [Internet]. 2015;38(6):506-14. Available from: https://iris.paho.org/handle/10665.2/18567
25. World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA [Internet]. 2013;310(20):2191-4. doi: https://doi.org/10.1001/jama.2013.281053
26. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) V5.0. 2017 [Internet]. Estados Unidos: National Cancer Institute; 2017 [Updated 2021 Apr 19]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_60
27. Mollica M, Newman SD. Breast cancer in African Americans: from patient to survivor. J Transcult Nurs [Internet]. 2014;25(4):334-40. doi: https://doi.org/10.1177/1043659614524248
28. Beelen LM, van Dishoeck AM, Tsangaris E, Coriddi M, Dayan JH, Pusic AL, et al. Patient-Reported Outcome Measures in Lymphedema: A Systematic Review and COSMIN Analysis. Ann Surg Oncol [Internet]. 2021;28(3):1656-68. doi: https://doi.org/10.1245/s10434-020-09346-0
29. Pommier B, Créac’h C, Beauvieux V, Nuti C, Vassal F, Peyron R, Robot-guided neuronavigated rTMS as an alternative therapy for central (neuropathic) pain: Clinical experience and long-term follow-up- Eur J Pain [Internet]. 2016;20(6):907-16. doi: https://doi.org/10.1002/ejp.815
30. Attal N, Poindessous-Jazat F, De Chauvigny E, Quesada C, Mhalla A, Ayache SS, et al. Repetitive transcranial magnetic stimulation for neuropathic pain: a randomized multicentre sham-controlled trial. Brain [Internet]. 2021;144(11):3328-39. doi: https://doi.org/10.1093/brain/awab208
31. Nurmikko T, MacIver K, Bresnahan R, Hird E, Nelson A, Sacco P. Motor Cortex Reorganization and Repetitive Transcranial Magnetic Stimulation for Pain-A Methodological Study. Neuromodulation [Internet]. 2016;19(7):669-78. doi: https://doi.org/10.1111/ner.12444
32. Ma S-M, Ni J-X, Li X-Y, Yang L-Q, Guo Y-N, Tang Y-Z. High-Frequency Repetitive Transcranial Magnetic Stimulation Reduces Pain in Postherpetic Neuralgia. Pain Med [Internet]. 2015;16(11):2162-70. doi: https://doi.org/10.1111/pme.12832
33. Ayache SS, Ahdab R, Chalah MA, Farhat WH, Mylius V, Goujon C, et al. Analgesic effects of navigated motor cortex rTMS in patients with chronic neuropathic pain. Eur J Pain [Internet]. 2016;20(9):1413-22. doi: https://doi.org/10.1002/ejp.864
34. Lefaucheur JP. Cortical neurostimulation for neuropathic pain: state of the art and perspectives. Pain [Internet]. 2016;157(Suppl 1):S81-9. doi: https://doi.org/10.1097/j.pain.0000000000000401
35. Quesada C, Pommier B, Fauchon C, Bradley C, Créac’h C, Vassal F, et al. Robot-Guided Neuronavigated Repetitive Transcranial Magnetic Stimulation (rTMS) in Central Neuropathic Pain. Arch Phys Med Rehabil [Internet]. 2018;99(11):2203-15.e1. doi: https://doi.org/10.1016/j.apmr.2018.04.013
36. Ratti MM, Gandaglia G, Sisca ES, Derevianko A, Alleva E, Beyer K, et al. A Systematic Review to Evaluate Patient-Reported Outcome Measures (PROMs) for Metastatic Prostate Cancer According to the COnsensus-Based Standard for the Selection of Health Measurement INstruments (COSMIN) Methodology. Cancers [Internet]. 2022;14(20):1-12. doi: https://doi.org/10.3390/cancers14205120
37. Andersson V, Bergman S, Henoch I, Simonsson H, Ahlberg K. Benefits of using the Brief Pain Inventory in patients with cancer pain: an intervention study conducted in Swedish hospitals. Support Care Cancer [Internet]. 2020;28(8):3721-9. doi: https://doi.org/10.1007/s00520-019-05200-6
38. Edirisinghe NP, Makuloluwa TR, Amarasekara TD, Goonewardena CSE. Evaluating psychometric properties of the Short Form Brief Pain Inventory Sinhala Version (SF BPI-Sin) among Sinhala speaking patients with cancer pain in Sri Lanka. BMC Psychol [Internet]. 2021;9(1):1-10. doi: https://doi.org/10.1186/s40359-021-00538-1
39. Cheng HL, Lopez V, Ching Lam S, To Leung AK, Li YC, Hung Wong K, et al. Psychometric testing of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx) subscale in a longitudinal study of cancer patients treated with chemotherapy. Health and Quality of Life Outcomes [Internet]. 2020;18(1):1-9. doi: https://doi.org/10.1186/s12955-020-01493-y
40. Sánchez-Pedraza R, Sierra-Matamoros FA, López-Daza DF. Validación colombiana de la escala FACT-B para medir la calidad de vida de pacientes con cáncer de mama. Rev colomb obstet ginecol [Internet]. 2012;63(3):196-206. doi: https://doi.org/10.18597/rcog.171
41. Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain [Internet]. 2014;155(12):2461-70. doi: https://doi.org/10.1016/j.pain.2014.09.020
42. Hodaj H, Payen J-F, Hodaj E, Dumolard A, Maindet C, Cracowski J-L, et al. Long-term treatment of chronic orofacial, pudendal, and central neuropathic limb pain with repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol [Internet]. 2020;131(7):1423-32. doi: https://doi.org/10.1016/j.clinph.2020.03.022
43. Kim JK, Park HS, Bae JS, Jeong YS, Jung KJ, Lim JY. Effects of multi-session intermittent theta burst stimulation on central neuropathic pain: A randomized controlled trial. NeuroRehabilitation [Internet]. 2020;46(1):127-34. doi: https://doi.org/10.3233/NRE-192958
44. Lawson McLean A, Frank S, Zafar N, Waschke A, Kalff R, Reichart R. Time course of the response to navigated repetitive transcranial magnetic stimulation at 10 Hz in chronic neuropathic pain. Neurol Res [Internet]. 2018;40(7):566-74. doi: https://doi.org/10.1080/01616412.2018.1453636
45. Yin S, Njai R, Barker L, Siegel PZ, Liao Y. Summarizing health-related quality of life (HRQOL): development and testing of a one-factor model. Popul Health Metr [Internet]. 2016;14:1-9. doi: https://doi.org/10.1186/s12963-016-0091-3
46. Cherif F, Zouari HG, Cherif W, Hadded M, Cheour M, Damak R. Depression Prevalence in Neuropathic Pain and Its Impact on the Quality of Life. Pain Res Manag [Internet]. 2020;2020. doi: https://doi.org/10.1155/2020/7408508
47. Torrance N, Ferguson JA, Afolabi E, Bennett MI, Serpell MG, Dunn KM, et al., Neuropathic pain in the community: more under-treated than refractory? Pain [Internet]. 2013;154(5):690-9. doi: https://doi.org/10.1016/j.pain.2012.12.022
48. Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci [Internet]. 2019;20(6):1-29. doi: https://doi.org/10.3390/ijms20061451