Exploring the Feasibility of a Comprehensive Screening for Voice and Swallowing Function in Post-Extubation Patients: A Pilot Study
Explorando la viabilidad de un tamizaje integral de la función de la voz y la deglución en pacientes postextubación: un estudio piloto
Fernanda Figueroa-Martínez, Adrián Castillo-Allendes , Karla Grunewaldt, Tamara Solís-Meza, Eric J. Hunter, Jeff Searl
Abstract
Objectives: This pilot study aimed to identify and test a battery of time-efficient and cost-effective voice and swallowing screening tools for post-extubated patients in Chile.
Methods: A panel of four experts selected and rated voice and swallowing screening tools. Seven measures were selected: smoothed cepstral peak prominence (CPPS) and maximum phonation time (MPT) for voice assessment, Volume-Viscosity Swallow Test (V-VST) for swallowing, voluntary and reflex peak cough flow for cough assessment, Eating Assessment Tool-10 (EAT-10), and Vocal Symptom Scale (VoiSS) for patient-reported outcomes. These tools were applied to four post-extubation patients within 48-72 hours post-hospital discharge, alongside the assessment of 17 matched controls.
Results: Post-extubation patients showed significantly shorter MPT, lower CPPS values, increased V-VST dysphagia signs, reduced voluntary peak cough flow, and more pronounced symptoms on both the VoiSS and EAT-10 compared to controls.
Limitations: The study had a modest sample size and relied solely on clinical screening tools.
Value This pilot study suggests a feasible and cost-effective approach to voice and swallowing screening for post-extubation patients, valuable in resource-constrained settings.
Conclusion: While these accessible tools are not gold-standard assessments, they offer valuable insights and can guide future research. This study underscores the potential of selected tools in facilitating early detection of voice and swallowing disorders in post-extubation patients.
Keywords
Endotracheal intubation; intensive care unit; cough; voice disorders; swallowing disorders.
Resumen
Objetivos: Este estudio piloto tuvo como objetivo identificar y probar una batería de herramientas de detección de problemas de voz y deglución que fueran eficientes en cuanto a tiempo y costo para pacientes chilenos postextubados.
Métodos: Un panel de cuatro expertos seleccionó y evaluó herramientas de detección de voz y deglución. Se seleccionaron siete medidas: prominencia de pico cepstral suavizado (CPPS) y tiempo máximo de fonación (TMF) para la evaluación de la voz, prueba de volumen-viscosidad (V-VST) para la deglución, flujo máximo voluntario y reflejo de la tos para evaluar la tos, Eating Assessment Tool-10 (EAT-10) y la Escala de Sintomas Vocales (ESV) para los resultados informados por los pacientes. Estas herramientas se aplicaron a cuatro pacientes postextubados (48-72 horas), junto con la evaluación de 17 controles pareados.
Resultados: Los pacientes postextubados mostraron un TMF y CPPS significativamente más bajos, aumento de los indicios de disfagia en la V-VST, reducción del flujo máximo de la tos y síntomas más pronunciados tanto en la ESV como en la EAT-10 en comparación con los controles.
Limitaciones: El estudio tuvo un tamaño de muestra reducida y se basó únicamente en herramientas de detección clínica.
Valor: Este estudio piloto sugiere un enfoque factible y rentable para la detección de problemas de voz y deglución en pacientes postextubados, valioso en entornos con recursos limitados.
Conclusión: Aunque ese abordaje no sustituye a las evaluaciones de referencia, ofrece información valiosa y puede guiar futuras investigaciones que busquen facilitar la detección temprana de los trastornos de la voz-deglución en pacientes postextubados.
Palabras clave
Intubación endotraqueal; cuidados intensivos; tos; trastornos de la voz; disfagia.
Introduction
The incidence of voice and swallowing issues in patients undergoing prolonged (>48 hours) invasive mechanical ventilation (IMV) is on the rise, driven not only by the increasing prevalence of respiratory and neuromuscular complications associated with chronic conditions in the aging population [1-3], but also by the emergence of COVID-19 [4]. While IMV provides vital respiratory support, it can also contribute to upper airway alterations that can lead to voice, swallowing, and cough disorders. Such patients have a higher risk of developing dysphonia (76%) and dysphagia (49%), which may persist beyond the hospital discharge. These conditions may impact their full functional recovery and quality of life [2,3,5-7]. For example, a significant incidence of respiratory disorders and cough dysfunction is reported in more than half of the subjects who receive IMV [3,5,6,8-10]. Even more alarming, post-IMV swallowing disorders are an independent predictor of mortality and directly related to the days of IMV and hospitalization [3,6,8]. A further consideration for this patient population is the possibility of developing intensive care unit acquired muscle weakness (ICUAW), which is a generalized muscle weakness developed during an ICU stay that may affect laryngeal and respiratory functions [11] and may persist for years after hospital discharge [12]. Although such difficulties may be expected in this population, they often remain unidentified [2,9]. Therefore, a timely assessment of communication and swallowing difficulties (including cough) prior to discharge is essential.
Tools to evaluate voice [13-17] and swallowing status have been developed and demonstrated to be effective [10,18-20]. However, common screening tools, such as videofluoroscopic swallowing study (VFSS), fiberoptic endoscopic evaluation of swallowing (FEES), instrumental voice assessment (stroboscopy and aerodynamic assessment), and digital spirometry evaluations, are expensive and difficult to access (and often unavailable) in many healthcare clinics worldwide. This lack of access to affordable and accessible screening tools limits clinical capabilities and care in many settings, including developing countries and underserved rural areas. This is especially true for patients who would benefit from cost-effective, easy-to-administer, and accessible screening tools.
The COVID-19 pandemic further highlighted the critical importance of timely speech-language pathology (SLP) screenings for voice and swallowing disorders [4,21]. For example, in Chile, despite the progress in healthcare reforms and advancements, there is still a need to improve service quality and access [22,23]. This situation highlights the need for feasible, cost-effective, and accessible approaches to assessing critical functions such as voice and swallowing in developing countries.
There were two aims of this pilot study: (1) identify a battery of user-friendly, time-efficient, and cost-effective screening tools for voice, swallow, and cough function; (2) apply the screening battery in a pilot study, comparing recently extubated patients to control participants who had not been intubated. By doing so, the study aims to contribute to the overarching goal of promoting equitable healthcare outcomes, regardless of individuals' geographical locations or socio-economic statuses, thereby advancing the cause of global healthcare equity. Due to the sequential nature of the two objectives, the methods, results, and a brief discussion regarding the first aim have been presented first, followed by the same components for aim two. This ordering was chosen to ensure a coherent and logical presentation of the study's progression and outcomes.
Study 1: identification of laryngeal function assessment tools
Methods
The selection of tools and measures that would be tested in Part 2 (pilot comparison of extubated to control participants) was completed in three steps. First, a group of experts who work extensively with voice and swallowing issues in patients who have been intubated was identified. This panel consisted of three highly experienced SLPs specialized in voice and swallowing disorders, with an average clinical experience of 10 years (range = 6 - 13 years of experience). Additionally, a respiratory therapist, well-versed in respiratory care, was included in the group. Significantly, all panel members possessed expertise in academia and research, bolstering their qualifications.
The second step was carried out by one of the co-authors (K.G.), who systematically conducted an extensive literature search to identify potential tools and measures pertinent to the assessment of voice and swallowing issues in post IMV patients. This comprehensive search encompassed academic databases (MEDLINE (via PubMed), Embase (OVID), Epistemonikos, and Lilacs), research articles, and relevant clinical guidelines. The search was adapted to each of the databases with terms such as “endotracheal intubation”, “voice assessment”, “swallowing assessment”, and “cough assessment”, using Boolean operators “AND” and “OR” to refine the results. Then, the expert panel rated the assessment tools and procedures, using a predetermined set of criteria, which included specific questions and ratings for validation, ease of application, affordability, measurability, and no requirement for extra certified training. To avoid bias, a blind response or vote by the individual panelists was conducted. Recommendation of an assessment tool or procedure was met when there was 80% agreement among the panelists’ responses.
The third step involved an independent rating process, meticulously designed to prevent any potential bias or bandwagon effect. Each panel member was individually engaged in a qualitative approach resembling a semi-structured interview. They were prompted to offer comprehensive insights into the identified tools and measures, addressing critical aspects such as feasibility, ease of utilization, personal history of using the tool as a screening method, and an analysis of its pros and cons. This method ensured a multifaceted evaluation, incorporating not only the objective aspects of the tools, but also the valuable practical experiences and perceptions of the clinical experts.
Lastly, following a rigorous thematic analysis, the research team collated the ratings, focusing on tools and measures that garnered a consensus of over 80% agreement among the clinical experts. The consensus underscored positive perceptions concerning the previously mentioned parameters, including cost-effectiveness, ease of application, and accessibility of the assessed tools and measures.
Results and brief discussion
Voice Screening
The clinical experts reached consensus on two measures to assess objective indicators of vocal function: (1) smoothed cepstral peak prominence (CPPS), a measure of vocal quality; and (2) maximum phonation time (MPT), a measure of phonatory efficiency. The CPPS was identified in the review process as a widely used acoustic measure of both steady vowels and connected speech that has been demonstrated to be sensitive to vocal quality and general degree of dysphonia [24,25]. CPPS is considered to be a robust measure for dysphonic voice characteristics during connected speech tasks that is repeatable and easy to get from freely available softwares, such as Praat or VoxPlot [25-27]. MPT was identified as a potentially useful measure in the screening protocol, serving as both an aerodynamic and perceptual screening of phonatory efficiency. It provides valuable information on the duration of sustained phonation, reflecting the coordination and control of the respiratory and laryngeal systems during vocalization. As an aerodynamic measure, MPT offers insights into glottal closure adequacy and overall vocal fold function [28-31]. It is hypothesized that the incorporation of these measures in the screening process would enhance the comprehensive evaluation of voice-related parameters, amplifying the clinical utility and diagnostic accuracy of the screening approach.
Swallowing Screening
The clinical experts’ ratings identified the Volume-Viscosity Swallow Test (V-VST) as a useful choice for the screening of dysphagia in patients, offering distinct advantages over other methods. As a bedside approach, the V-VST serves as a valuable screening tool, enabling the identification of signs indicative of altered safety and efficiency during the intake process. Notably, the V-VST is easily accessible and time-efficient. With a sensitivity of 88.2%, the V-VST demonstrates high diagnostic accuracy in screening for oropharyngeal dysphagia. The clinical experts indicated that by incorporating the V-VST as a part of the screening protocol, the evaluation process could be efficient while ensuring reliable identification of swallowing disorders, thus facilitating early intervention and improved patient outcomes [20]. This test considers the use of food boluses of different volumes and viscosities with the incorporation of pulse oximetry. During each intake, the possible signs of alteration of the safety and efficiency of swallowing are registered.
Cough Screening
An analog peak flow meter (e.g., Mini-Wright Peak Flow Meter) was identified by the clinical experts' ratings as an approach for cough screening that would be more accessible in many parts of the world, because it is less expensive than digital spirometry, thereby offering practicality without compromising accuracy. The device can be purchased online from a variety of distributors for approximately $35 USD. By employing the analog peak flow meter, two crucial measures (i.e., voluntary peak cough flow, vPCF; reflex peak cough flow, rPCF) can be readily obtained. These measures serve as reliable indicators of cough strength and provide valuable insights into the respiratory capabilities of individuals [1]. Based on the assessment of the clinical experts, the adoption of the analog peak flow meter as a part of the screening protocol would enable clinicians to conduct efficient and cost-effective evaluations of cough function, facilitating early identification of abnormalities and appropriate intervention strategies [1,32,33].
Patient Reported Outcome Measures
Two Patient-Reported Outcome Measures (PROMs) were identified for evaluating the self-perception of voice and swallowing function: the Eating Assessment Tool-10 (EAT-10) and the Vocal Symptom Scale (VoiSS). These scales were identified based on their accessibility, ease of administration, and affordability. Further, these scales have been validated for the Chilean population, which distinguishes them as the only validated self-perception measures available for this specific population [34,35]. The EAT-10 is a concise questionnaire (10 questions) that individuals can easily complete to identify swallowing impairment based on the individual's perspective (cut-off score of 7 points) [35]. The VoiSS is a comprehensive 30-item self-report survey that provides a robust screening of vocal symptoms. A cut-off score of 15 points or higher indicates the presence of vocal impairment with a significant impact on the individual's quality of life [34].
Study 2: pilot data assessing recently extubated patients
Methods
Participants
The study participants included extubated individuals who were discharged from the university hospital. The inclusion criteria for the patient group were (1) between 18 and 80 years of age, (2) intubation and mechanical ventilation for a period exceeding 48 hours, (3) baseline functional independence after intubation for activities of daily living, as determined by achieving a Barthel Index score of ≥70 points, (4) Mini-Mental State Exam score above 24, thereby ensuring their capacity to adhere to the study protocol, and (5) hospital discharge between 48-72 hours before the time of their participation in the pilot study. Exclusion criteria were (1) a history of tracheostomy use, neuromuscular pathology, smoking, or recent cardiorespiratory arrest, and (2) a documented clinical history of voice and/or swallowing abnormalities prior to hospitalization. This study received approval from the Scientific Ethics Committee of the Faculty of Medicine at the Pontificia Universidad Católica de Chile.
A group of healthy controls were recruited to match the age and sex (biological factor) of the post-IMV patient cohort. These control participants were adults recruited from the university hospital who exhibited normal voice and swallowing functions, based on a clinical interview conducted by two members of the research group (A.C. and F.F.), in which participants self-reported no communication or swallowing difficulties. They also self-reported no prior history of voice or swallowing disorders, intubation, tracheostomy tube use, neuromuscular pathology, smoking, or cardiac arrest.
Procedures
Voice recordings were made in a soundproof room with the subject seated at a background noise below 35 dBA, using a portable digital recorder (Tascam model DR40; 44 kHz, 16 bits, wav) placed 45° and 20 cm from the mouth. To obtain MPT, participants were recorded while producing three repetitions of /a/ sustained at comfortable intensity for the maximum duration possible. Praat Software (
The swallowing screening was performed by one of the researchers (T.S.) using the V-VST. Participants were asked to swallow three viscosities, starting with nectar, then pudding, and finally liquid, in single boluses of 5, 10, and 20 ml that were administered through a syringe. Changes in voice quality, coughing, or decreased oxygen saturation (≥3%) were registered as signs of impaired safety as specified in the V-VST. A failed V-VST occurred if any of these signs were present [20].
Cough assessment was completed using a handheld analog spirometer (Mini-Wright Peak Flow Meter). For vPCF, participants were instructed to "Cough as if something went down the wrong pipe." The participant attempted the cough at least three times. If the measurements did not differ by more than 20 L/min between the three trials, then the highest value of the three was recorded as the vPCF. If there was a difference of more than 20 L/min, the maneuver was repeated up to a maximum of 8 times with the highest value being registered. To assess rPCF, procedures proposed by Curtis and Troche for Handheld Cough Testing were applied [32]. For this task, subjects were instructed to "Inhale and exhale through the mouth, and cough, if necessary." This was repeated three times, and the highest value was registered. For the patient reported outcome measures, both the VoiSS and EAT-10 were completed as paper-pencil questionnaires. All participants were able to complete the forms independently.
Statistical analysis
Statistical analysis was performed with R version 4.0.5, using a significance level of 0.05. Quantitative variables were described by mean and standard deviation (SD), while absolute and relative frequencies were reported for categorical variables. The Shapiro Wilk test was applied to determine the type of distribution of the variables. The t-test was used to compare quantitative variables with normal distribution (CPPS, MPT, and rPCF), while the nonparametric Mann-Whitney U test was used for those without normal distribution (vPCF). Chi-squared test was used to compare dichotomous variables (V-VST, VoiSS and EAT-10). Finally, Graphpad Prism 9 was used to develop the graphics.
Results
Participants
For the pilot study, four recently discharged patients were in the post-extubation group (N=4) and 17 age- and sex-matched adults were in the control group (N=17). The post-extubation group had a median age of 56.5 years and an interquartile range (IQR) from 30 - 69.5 years. Sex distribution in this group was evenly represented (2 m, 2 f). The control group had a median age of 49 years and IQR from 29 -70.5 years. Within this control group, 58.8% of participants were females, effectively emulating the distribution observed in the post-extubation group (see Table 1).
Table 1. Physical characteristics and hospitalization data of the subjects in the control group.
| Subject | Age (years) | Biological Sex | Adm Dx | IMV days | ICU days | Hosp days | CS use |
|---|---|---|---|---|---|---|---|
| 1 | 73 | M | Uremic syndrome | 13 | 13 | 18 | No |
| 2 | 59 | F | Obstructive shock | 3 | 9 | 15 | No |
| 3 | 54 | M | Septic shock | 21 | 37 | 60 | No |
| 4 | 22 | F | Hepatic infarction | 4 | 15 | 49 | No |
| Median (IR) | 56.5 (54-59) | - | - | 8.5 (4-13) | 14 (13-15) | 33.5 (18-49) | - |
Note. M, male; F, female; Adm Dx, admitting diagnosis; IMV, invasive mechanical ventilation; ICU, intensive care unit; Hosp., Hospitalization; CS, corticosteroid; IR, interquartile range.
Vocal function
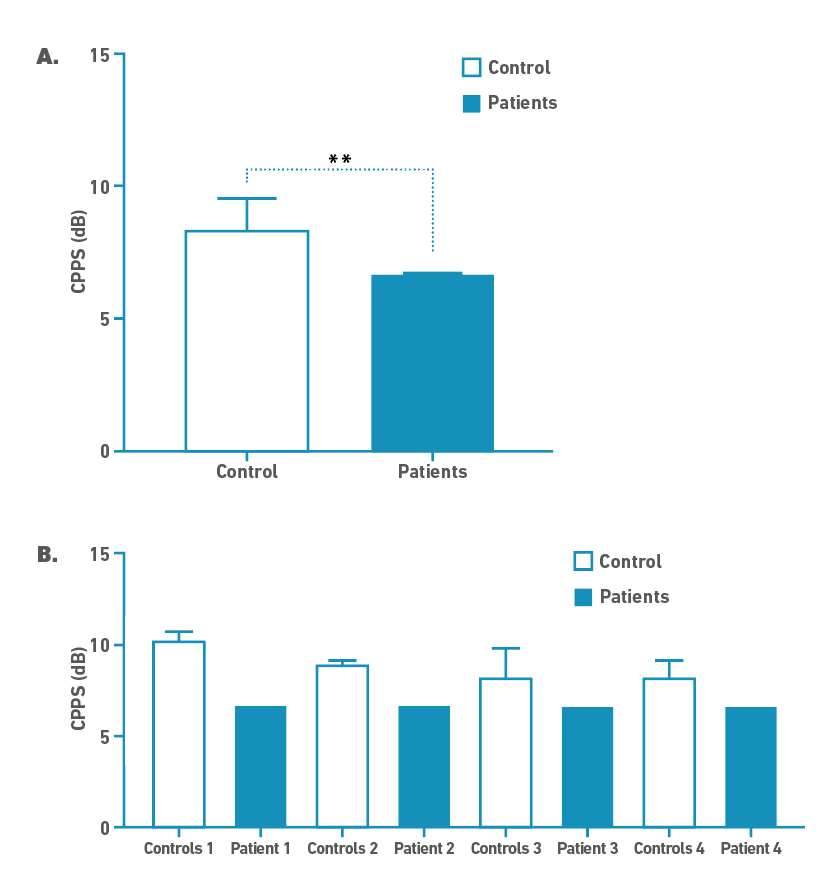
Table 2 includes descriptive statistics and results for CPPS and MPT. The t-test indicated that MPT was significantly shorter for the post-IMV patient group than the controls (t = 3.718; df = 19; p-value = 0.002). In voice quality acoustic assessment, the post-IMV group showed significantly lower CPPS than the control group (t = 3.185; df = 19, p-value = 0.004), as it can be seen in Figure 1.
Table 2. Median, Interquartile range, p-value between both groups respective vocal function measures.
| Control group (IQR) | Post IMV group (IQR) | p-value | |
|---|---|---|---|
| MPT (s) | 22.00(20.00-26.00) | 13.50(10.00-15.00) | 0.0015* |
| CPPS | 8.34(8.00-9.25) | 6.70(6.66-6.74) | 0.0040* |
Note. *Significant values. The Mann-Whitney U test was applied for the variables indicated.
Figure 1. A.) Median differences between control and IMV group (case) in CPPS; **- p<0.05. B.) CPPS differences between each patient and their age and gender matched controls.
Swallowing function and Cough
For the clinical screening of dysphagia, the Chi-squared test indicated that the proportion of post-IMV patients with dysphagia indicated by the V-VST was significantly greater than the proportion of controls (χ2 = 9.395, df = 1, p-value = 0.002), as shown in Table 3.
Table 3. Frequency and p-value for subjects who showed signs of impaired swallowing safety on V-VST, self-assessment status related to voice (VoiSS), and deglutition (EAT-10).
| Controls n (%) | Adults who received IMV n (%) | p value | |
|---|---|---|---|
| V-VST | 0 (0.00) | 2 (50.00) | 0.002* |
| VoiSS | 2 (11.76) | 3 (75.00) | 0.008* |
| EAT-10 | 0 (0.00) | 1 (100.00) | 0.035* |
Note. Note. n-frequency of subjects, % - percentage of subjects, *significant values. A chi-squared test was applied for the variables indicated.
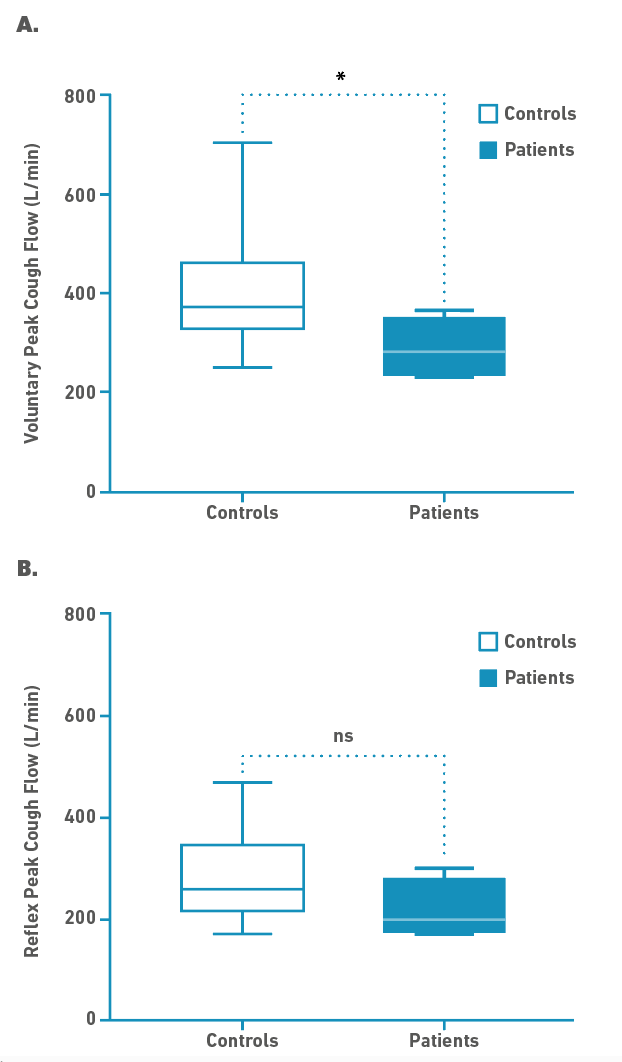
Regarding cough, the post-IMV group showed significantly lower vPCF values (U = 11, p-value = 0.038) with a median (IQR) of 280 (233-350) L/min compared to 370 (325-460) L/min for the control group. The median rPCF was lower for the post-IMV group (280 L/min) relative to the controls (200 L/min), but this difference was not statistically significant (t = 1.826, df = 6.236, p-value = 0.116), as it can be seen in Figure 2.
Figure 2. Median differences between control and IMV group (case) measured in L/min. A.) Voluntary Peak Cough Flow (vPCF); *- p<0.05. B.) Reflex Peak Cough Flow (rPCF).
Patient Reported Outcomes for Voice and Swallowing
The post-IMV group had significantly greater voice symptoms reported on the VoiSS questionnaire than the control group as indicated by the statistically significant Chi-squared test (χ2 = 7.138, df = 1, p-value = 0.008). Likewise, the post-IMV group had significantly greater swallowing issues reported on the EAT-10 questionnaire than the controls (χ2 = 4.463, df = 1, p-value = 0.035), as shown in Table 3.
Discussion
This study aimed to address the urgent need for a practical and cost-effective approach to assessing voice and swallowing function in post-extubation patients, thereby assisting clinicians, including medical speech-language pathologists, in making informed clinical decisions, enhancing patient care, and improving overall quality of life for individuals recovering from intubation.
The results revealed significant vocal impairments in the post-extubation group, including shorter maximum phonation time (MPT) and lower smoothed cepstral peak prominence (CPPS), which indicated a compromised vocal quality. Normal MPT values reported in the literature range from 15 to 35 seconds in adult patients [29], which corresponds with the results from the control participants in the present study. In contrast, the post-extubation group exhibited significantly lower MPT (13.5 sec; p<0.05). These findings are consistent with previous evaluations of MPT in post-extubation patients [17,31]. A lower MPT could be explained by defective glottal closure, which causes air leakage through the vocal folds [17]. Although MPT is not a diagnostic tool for laryngeal pathology, it has been demonstrated to be a reliable indicator of phonatory efficiency [28]. The results of the present proposal and pilot study show that MPT differentiated post-extubation patients from controls and is also a simple and accessible tool that might be useful to help detect voice disorders in this population.
Like MPT, the CPPS results demonstrated a difference between the patients and controls. Typically, lower CPPS values are associated with increasingly noisy or dysphonic signals [36-38]. There are currently no universal normative CPPS values, partly because there are likely differences related to the specific speech sample that is utilized. For example, in a group of vocally healthy Spanish speakers, normative values of CPPS ranged from 7.8 to 11.3 dB, depending on the specific phrase that was analyzed [39]. Some have suggested a cut-off threshold of 9.33 dB for connected speech (Rainbow Passage as measured in Praat) to determine whether a person has a higher probability of having a voice disorder [24]. Using this threshold would mean that the control participants in the current study were slightly below the threshold. Until more definitive norms are established that consider the specific connected speech sample, the language being spoken, and other variables (e.g., age, sex), it may not be possible to confidently state a threshold CPPS value for use in screening post-extubation patients. Given that there was a statistical difference between the patients and controls in this study, it seems worthwhile to further explore CPPS as a candidate measure for such a screening battery, although a substantially larger group of control and experimental participants will be needed from the Chilean population.
In summary, the post-IMV group presented markedly breathier voices and phonatory inefficiency [40], which are signs that are often overlooked and which could translate into long-term dysphonia [2]. Further, these signs do not only impact the voice quality and function but also swallowing and cough.
Regarding swallowing, post-IMV patients demonstrated signs of a compromised swallowing (as evidenced by the Volume-Viscosity Swallow Test [V-VST]) and reduced cough strength (as indicated by lower peak cough flow [PCF] values). Post-IMV swallowing impairments can be explained by factors such as a limited laryngeal ascent and impaired sensitivity [2]. Other authors have also reported a decrease in both voluntary peak cough flow (vPCF) and reflex peak cough flow (rPCF) a few hours after extubation [41]. This reduced cough strength can be explained by a reduced subglottal pressure and defective glottal closure [42,43].
These results highlight the importance of an early assessment and diagnosis in this population, especially taking cough function into consideration as it has been reported in the literature as a common alteration in post-extubation patients that can highly compromise swallowing safety, impacting patients' mortality and quality of life [11,12,32,33].
Most of the previously mentioned alterations on the overall larynx function are related to a defective glottal closure that can be explained by anatomical and/or muscular reasons [30]. In previous studies, thorough evaluation of larynx damage in this population have shown varying degrees of iatrogenic damage due to the prolonged presence of the endotracheal tube [44], such as oedema, erythema, haematoma, granuloma, and nerve conduction damage, which can result in phonatory and respiratory issues [40]. While some of these lesions could heal in the span of a few days or weeks, nerve damage could take months to recover. Vocal fold palsy is one of the most severe post-IMV laryngeal damage, as it can severely increase posoperative morbidity and mortality, being associated with complications like dysphagia, dysphonia, and a poor respitarory control [45]. Intensive care unit-acquired weakness (ICUAW) also affects the laryngeal and vocal fold muscles, impairing voice, and swallowing. Recovery may begin in a few weeks or months, but complete recovery may take longer, as weakness can persist for up to two years after discharge and has also been shown to increase the risk of mortality within one year [11,12,46]. Therefore, an early assessment of the correct functioning of the larynx and other structures that might be affected by weakness is a priority for these patients.
The self-perception questionnaires further supported the objective measurement results, with post-IMV patients reporting more voice symptoms and swallowing issues than the control group. These findings highlight the critical importance of early assessment and intervention to address the vocal and swallowing impairments experienced by individuals recovering from intubation. Additionally, incorporating self-report tools into the assessment process provided valuable insights into patients' self-perceived voice and swallowing abilities. This insight enables clinicians to tailor interventions to patients' individual needs, significantly enhancing their effectiveness and improving patient satisfaction.
As expected, post-IMV patients differed from controls on nearly all measures included in the battery. However, larger-scale studies are needed to verify these findings. Nevertheless, the current results identify a preliminary battery of simple and easy-to-use tools for screening voice and swallowing that can be assessed in the future. These tools can be used in several ways, including more feasible longitudinal studies that track patients' progression over time.
Larger-scale studies are warranted to validate the effectiveness and applicability of this approach. Future research could also focus on validating specific tools and comparing their sensitivity and specificity with established instruments such as VFSS or FEES. Such studies would ultimately enhance the standard of care for post-IMV patients by facilitating early detection and appropriate management of voice and swallowing disorders.
Clinical Implications
The findings of this study have significant implications for healthcare professionals involved in the care of extubated individuals, especially in resource-limited settings. Early voice and swallowing assessment are critical, and the proposed screening approach is practical and cost-effective. The pilot data demonstrates the effectiveness of the selected assessment tools in identifying voice and swallowing disorders in post-IMV patients.
The study found significant differences in vocal status, swallowing function, and self-perception between post-IMV patients and the control group. These findings highlight the importance of early detection and intervention to improve patient outcomes and quality of life. By promptly identifying and addressing voice and swallowing issues, healthcare professionals can optimize patient care, improve treatment outcomes, and enhance the overall well-being of post-IMV patients.
The proposed screening approach is holistic and comprehensive, yet easily accessible and inexpensive. It does not advocate for a rigid or inflexible assessment approach, but instead aims to raise awareness of the possibility of conducting such assessments using readily available tools. This enhances accessibility to assessments and encourages healthcare professionals to adopt a broader perspective on patient care, recognizing the integral role of addressing voice and swallowing disorders in overall well-being.
This is the first study to comprehensively explore the assessment of laryngeal functions, including voice, cough, and swallowing, while also considering the perspectives of patients in the post-IMV patient population. This provides valuable insights into the field and pushes the boundaries of assessment practices. It contributes to the ongoing advancement of clinical knowledge and paves the way for further research and innovation in the field of voice and swallowing assessment in extubated patients.
The proposed tools have the potential to be used in regions with limited clinical resources. While they do not replace specialized tests such as videofluoroscopic swallowing study (VFSS) or fiberoptic endoscopic evaluation of swallowing (FEES), they can provide valuable preliminary information through early, rapid, and cost-effective (≤$350 USD) screening. This is particularly significant in developing countries and underserved rural areas, where gold standard assessments may be unavailable.
Limitations
The primary limitation of this study is the small number of participants in the post-IMV group. This pilot study was designed to gather preliminary data on the feasibility of the proposed screening protocol and to determine whether it warrants further investigation in a larger study. As such, it is not possible to draw definitive conclusions about the effectiveness of the tools in identifying voice and swallowing disorders in post-IMV patients. Additionally, the swallowing assessment used in the study (V-VST) is a screening tool and does not provide information about the swallowing pathophysiology of the users. Therefore, patients with suspected post-extubation dysphagia would still need to be referred for an objective evaluation using VFSS or FEES.
Another limitation is that the EAT-10 questionnaire is not designed to assess quality of life. It is a screening tool validated in Chile to detect dysphagia risk in older adults [35].
Despite these limitations, the study provides valuable preliminary data on the potential utility of the proposed screening protocol in identifying voice and swallowing disorders in post-IMV patients. Further research with a larger sample size and a more comprehensive swallowing assessment is warranted to validate the protocol and establish specific threshold values and cut-off scores.
Conclusion
This pilot study has presented a practical and cost-effective approach for screening voice and swallowing function in post-extubation patients. Our findings suggest that selected tools, such as MPT and the V-VST, have the potential to detect voice and swallowing disorders early.
Post-IMV patients exhibited significantly impaired vocal quality, as evidenced by a shortened MPT and reduced CPPS. These accessible tools, particularly the MPT, show promise for early detection of voice disorders.
Additionally, our study revealed compromised swallowing safety, as demonstrated by the V-VST, and reduced cough strength, indicated by lower peak cough flow (PCF) values, in post-IMV patients. These findings underscore the importance of early assessment and intervention to improve patient outcomes.
The incorporation of self-perception questionnaires has enhanced patient-centered care by providing valuable insights into patients' voice and swallowing abilities. This holistic approach ensures a more comprehensive understanding of patient needs, allowing for tailored interventions and ultimately improving patient satisfaction and care quality.
This pilot study has presented a practical and cost-effective alternative to gold-standard assessments for screening voice and swallowing function in extubated patients, especially in resource-limited settings. The findings suggest that the selected tools are feasible and effective in identifying voice and swallowing disorders early. Further research is warranted to validate these tools and expand our understanding of voice and swallowing assessment in extubated patients.
References
1. Ambrosino N, Vitacca M. The patient needing prolonged mechanical ventilation: A narrative review. Multidiscip Respir Med. 2018;13(1):1-10. doi: https://doi.org/10.1186/s40248-018-0118-7
2. Brodsky MB, Levy MJ, Jedlanek E, Pandian V, Blackford B, Price C, et al. Laryngeal Injury and Upper Airway Symptoms after Oral Endotracheal Intubation with Mechanical Ventilation During Critical Care. Crit Care Med [Internet]. 2018 Dec;46(12):2010-7.doi: https://doi.org/10.1097/CCM.0000000000003368
3. Zuercher P, Schenk N V., Moret C, Berger D, Abegglen R, Schefold JC. Risk Factors for Dysphagia in ICU Patients After Invasive Mechanical Ventilation. Chest [Internet]. 2020;158(5):1983-91. doi: https://doi.org/10.1016/j.chest.2020.05.576
4. Miles A, McRae J, Clunie G, Gillivan-Murphy P, Inamoto Y, Kalf H, et al. An International Commentary on Dysphagia and Dysphonia During the COVID-19 Pandemic. Dysphagia [Internet]. 2022;37(6):1349-74. doi: https://doi.org/10.1007/s00455-021-10396-z
5. Shinn JR, Kimura KS, Campbell BR, Sun Lowery A, Wootten CT, Garrett CG, et al. Incidence and Outcomes of Acute Laryngeal Injury After Prolonged Mechanical Ventilation*. Crit Care Med [Internet]. 2019 Dec;47(12):1699-706. doi: https://doi.org/10.1097/CCM.0000000000004015
6. Schefold JC, Berger D, Zürcher P, Lensch M, Perren A, Jakob SM, et al. Dysphagia in Mechanically Ventilated ICU Patients (DYnAMICS). Crit Care Med [Internet]. 2017 Dec;45(12):2061-9. doi: https://doi.org/10.1097/CCM.0000000000002765
7. Dawson C, Clunie G, Evison F, Duncan S, Whitney J, Houchen-Wolloff L, et al. Prevalence of swallow, communication, voice and cognitive compromise following hospitalisation for COVID-19: the PHOSP-COVID analysis. BMJ open Respir Res. 2023;10(1). doi: https://doi.org/10.1136/bmjresp-2023-001647
8. Campbell BR, Shinn JR, Kimura KS, Lowery AS, Casey JD, Ely EW, et al. Unilateral Vocal Fold Immobility after Prolonged Endotracheal Intubation. JAMA Otolaryngol - Head Neck Surg. 2020;146(2):160-7. doi: https://doi.org/10.1001/jamaoto.2019.3969
9. Colton House J, Noordzij JP, Murgia B, Langmore S. Laryngeal injury from prolonged intubation: A prospective analysis of contributing factors. Laryngoscope [Internet]. 2011 Mar;121(3):596-600. doi: https://doi.org/10.1002/lary.21403
10. Scheel R, Pisegna JM, McNally E, Noordzij JP, Langmore SE. Endoscopic Assessment of Swallowing After Prolonged Intubation in the ICU Setting. Ann Otol Rhinol Laryngol [Internet]. 2016 Jan 26;125(1):43-52. doi: https://doi.org/10.1177/0003489415596755
11. Hermans G, Van den Berghe G. Clinical review: Intensive care unit acquired weakness. Crit Care [Internet]. 2015;19(1):1-9. doi: http://dx.doi.org/10.1186/s13054-015-0993-7
12. Ballve LPD, Dargains N, Inchaustegui JGU, Bratos A, Percaz M de los M, Ardariz CB, et al. Weakness acquired in the intensive care unit. Incidence, risk factors and their association with inspiratory weakness. Observational cohort study. Rev Bras Ter Intensiva [Internet]. 2017;29(4):466-75. Available from: https://www.scielo.br/j/rbti/a/48Jbmf4fHt7Rd9VZPYHXwmC/?lang=en#
13. Patel R, Awan SN, Barkmeier-Kraemer J, Courey M, Deliyski D, Eadie T, et al. Recommended Protocols for Instrumental Assessment of Voice: American Speech- Language-Hearing Association Expert Panel to Develop a Protocol for Instrumental Assessment of Vocal Function Rita. Am J Speech-Language Pathol. 2018;27(3):887-905. doi: https://doi.org/10.1044/2018_AJSLP-17-0009
14. Van der Meer G, Ferreira Y, Loock JW. The S/Z ratio: A simple and reliable clinical method of evaluating laryngeal function in patients after intubation. J Crit Care [Internet]. 2010;25(3):489-92. doi: http://dx.doi.org/10.1016/j.jcrc.2009.11.009
15. Lim J-Y, Yoo Y-H, Park C-H, Joa K-L, Jung H-Y. Use of the maximal phonation test for the screening of dysphagia in stroke patients: a preliminary study. Eur J Phys Rehabil Med [Internet]. 2020 Feb;56(1):41-6. doi: https://doi.org/10.23736/S1973-9087.19.05818-0
16. Peterson EA, Roy N, Awan SN, Merrill RM, Banks R, Tanner K. Toward validation of the cepstral spectral index of dysphonia (CSID) as an objective treatment outcomes measure. J Voice [Internet]. 2013;27(4):401-10. doi: http://dx.doi.org/10.1016/j.jvoice.2013.04.002
17. Asiaee M, Vahedian-azimi A, Atashi SS, Keramatfar A, Nourbakhsh M. Voice Quality Evaluation in Patients With COVID-19: An Acoustic Analysis. J Voice [Internet]. 2020;36(6):879.e13-879.e19. doi: https://doi.org/10.1016/j.jvoice.2020.09.024
18. Lascarrou JB, Boisrame-Helms J, Bailly A, Le Thuaut A, Kamel T, Mercier E, et al. Video Laryngoscopy vs Direct Laryngoscopy on Successful First-Pass Orotracheal Intubation Among ICU Patients. JAMA [Internet]. 2017 Feb 7;317(5):483. doi: https://doi.org/10.1001/jama.2016.20603
19. Clavé P, Arreola V, Romea M, Medina L, Palomera E, Serra-Prat M. Accuracy of the volume-viscosity swallow test for clinical screening of oropharyngeal dysphagia and aspiration. Clin Nutr [Internet]. 2008 Dec;27(6):806-15. doi: https://doi.org/10.1016/j.clnu.2008.06.011
20. Rofes L, Arreola V, Clavé P. The Volume-Viscosity Swallow Test for Clinical Screening of Dysphagia and Aspiration. In: Stepping Stones to Living Well with Dysphagia [Internet]. Barcelona: Institute Workshop; 2012. p. 33-42. doi: https://doi.org/10.1159/000339979
21. Vega Rodríguez YE, Torres Rodríguez AM, del Campo Rivas MN. Análisis del Rol del Fonoaudiólogo(a) en el Sector Salud en Chile. Cienc Trab [Internet]. 2017 Aug;19(59):76-80. doi: https://doi.org/10.4067/S0718-24492017000200076
22. Paraje G, Vásquez F. Health equity in an unequal country: the use of medical services in Chile. Int J Equity Health [Internet]. 2012;11(1):81. doi: https://doi.org/10.1186/1475-9276-11-81
23. Koch KJ, Cid Pedraza C, Schmid A. Out-of-pocket expenditure and financial protection in the Chilean health care system-A systematic review. Health Policy (New York) [Internet]. 2017;121(5):481-94. doi: http://dx.doi.org/10.1016/j.healthpol.2017.02.013
24. Murton O, Hillman R, Mehta D. Cepstral peak prominence values for clinical voice evaluation. Am J Speech-Language Pathol. 2020;29(3):1596-607. doi: https://doi.org/10.1044/2020_AJSLP-20-00001
25. Watts CR, Awan SN, Maryn Y. A Comparison of Cepstral Peak Prominence Measures from Two Acoustic Analysis Programs. J Voice [Internet]. 2017;31(3):387.e1-387.e10. doi: http://dx.doi.org/10.1016/j.jvoice.2016.09.012
26. Baker CP, Sundberg J, Purdy SC, Rakena TO, Leão SH d. S. CPPS and Voice-Source Parameters: Objective Analysis of the Singing Voice. J Voice. 2022;S0892-1997(21)00433-1. doi: https://doi.org/10.1016/j.jvoice.2021.12.010
27. Lopes LW, Sousa ES da S, da Silva ACF, da Silva IM, de Paiva MAA, Vieira VJD, et al. Cepstral measures in the assessment of severity of voice disorders. Codas. 2019;31(4):1-8. doi: https://doi.org/10.1590/2317-1782/20182018175
28. Speyer R, Bogaardt HCA, Passos VL, Roodenburg NPHD, Zumach A, Heijnen MAM, et al. Maximum Phonation Time: Variability and Reliability. J Voice [Internet]. 2010 May;24(3):281-4. doi: https://doi.org/10.1016/j.jvoice.2008.10.004
29. Hirano, M., Koike, Y., & von Leden, H. Maximum Phonation Time and Air Usage During Phonation. Folia Phoniatr Logop. 1968;20(4):185-201. doi: https://doi.org/10.1159/000263198
30. Fujimaki Y, Tsunoda K, Kobayashi R, Tonghyo C, Tanaka F, Kuroda H, et al. Independent exercise for glottal incompetence to improve vocal problems and prevent aspiration pneumonia in the elderly: A randomized controlled trial. Clin Rehabil. 2017;31(8):1049-56. doi: https://doi.org/10.1177/0269215516673208
31. Hamdan AL, Sibai A, Rameh C, Kanazeh G. Short-Term Effects of Endotracheal Intubation on Voice. J Voice. 2007 Nov 1;21(6):762-8. doi: https://doi.org/10.1016/j.jvoice.2006.06.003
32. Curtis JA, Troche MS. Handheld Cough Testing: A Novel Tool for Cough Assessment and Dysphagia Screening. Dysphagia [Internet]. 2020;35(6):993-1000. doi: https://doi.org/10.1007/s00455-020-10097-z
33. Bianchi C, Baiardi P, Khirani S, Cantarella G. Cough peak flow as a predictor of pulmonary morbidity in patients with dysphagia. Am J Phys Med Rehabil. 2012;91(9):783-8. doi: https://doi.org/10.1097/PHM.0b013e3182556701
34. Ruston FC, Moreti F, Vivero M, Malebran C, Behlau M. Cross-cultural adaptation of the Chilean version of the Voice Symptom Scale - VoiSS. Codas. 2016;28(5):625-33. doi: https://doi.org/10.1590/2317-1782/20162015249
35. Fernández-Rosati J, Lera L, Fuentes-López E, Albala C. Validez y confiabilidad del cuestionario Eating Assessment Tool 10 (EAT-10) para detectar disfagia en adultos mayores chilenos. Rev Med Chil [Internet]. 2018 Sep;146(9):1008-15. doi: https://doi.org/10.4067/s0034-98872018000901008
36. Maryn Y, Weenink D. Objective dysphonia measures in the program praat: Smoothed cepstral peak prominence and acoustic voice quality index. J Voice [Internet]. 2015;29(1):35-43. doi: http://dx.doi.org/10.1016/j.jvoice.2014.06.015
37. Hillenbrand J, Houde RA. Acoustic correlates of breathy vocal quality: Dysphonic voices and continuous speech. J Speech, Lang Hear Res. 1996;39(2):311-21. doi: https://doi.org/10.1044/jshr.3902.311
38. Heman-Ackah YD, Michael DD, Goding GS. The relationship between cepstral peak prominence and selected parameters of dysphonia. J Voice. 2002;16(1):20-7. doi: https://doi.org/10.1016/S0892-1997(02)00067-X
39. Núñez-Batalla F, Cartón-Corona N, Vasile G, García-Cabo P, Fernández-Vañes L, Llorente-Pendás JL. Validation of the Measures of Cepstral Peak Prominence as a Measure of Dysphonia Severity in Spanish-Speaking Subjects. Acta Otorrinolaringol. 2019;70(4):222-8. doi: https://doi.org/10.1016/j.otoeng.2018.04.005
40. Arviso LC, Klein AM, Johns MM. The management of postintubation phonatory insufficiency. J Voice. 2012 Jul 1;26(4):530-3. doi: https://doi.org/10.1016/j.jvoice.2010.10.022
41. Kallesen M, Psirides A, Huckabee ML. Comparison of cough reflex testing with videoendoscopy in recently extubated intensive care unit patients. J Crit Care [Internet]. 2016;33(2016):90-4. doi: http://dx.doi.org/10.1016/j.jcrc.2016.02.004
42. Britton D, Roeske A, Ennis SK, Benditt JO, Quinn C, Graville D. Utility of Pulse Oximetry to Detect Aspiration: An Evidence-Based Systematic Review. Dysphagia [Internet]. 2018;33(3):282-92. doi: https://doi.org/10.1007/s00455-017-9868-1
43. Pitts T. Airway protective mechanisms. Lung. 2014;192(1):27-31. doi: https://doi.org/10.1007/s00408-013-9540-y
44. Yoshiyuki H, Fuller BF. Selected acoustic characteristics of voices before intubation and after extubation. J Speech Hear Res. 1990;33(3):505-10. doi: https://doi.org/10.1044/jshr.3303.505
45. Sariego J. Vocal Fold Hypomobility Secondary to Elective Endotracheal Intubation: A General Surgeon’s Perspective. J Voice [Internet]. 2010;24(1):110-2. doi: http://dx.doi.org/10.1016/j.jvoice.2008.05.001
46. Lipshutz AKM, Gropper MA. Acquired neuromuscular weakness and early mobilization in the intensive care unit. Anesthesiology. 2013;118(1):202-15. doi: https://doi.org/10.1097/ALN.0b013e31826be693