Neuromuscular Alterations Associated with COVID-19. A Scientific Literature Review
Alteraciones neuromusculares asociadas a la COVID-19. Revisión de la literatura científica
Eulalia Maria Amador-Rodero , Priscila Mishelle Bartolo Gómez, Fátima del Carmen Carrasco Ferrer, Nancy Paola Ochoa Castillo, Leslie Piedad Montealegre Esmeral, Roberto Carlos Rebolledo Cobos
Abstract
Introduction: The COVID-19 pandemic has brought short, medium, and long-term consequences on the quality of life of those affected. Among the complications are those related to the involvement of the nervous system and the structures involved in body movement, with sequelae that may be transitory and/or definitive, and require rehabilitation.
Objective: Identify the neuromuscular alterations that affect body movement, associated with COVID-19.
Material and methods: A search was made for observational works published in the SCOPUS, PubMed, EBSCO, and Nature databases between January 2020 and June 2022 under the PRISMA methodology, to answer the PICO question: what are the neuromuscular alterations that can potentially affect movement, associated with COVID-19? The established filters were type of study, language, age, availability, publication dates. The MeSH terms were SARS-Cov-2, COVID-19, Long Covid, Motor Activity Neuromuscular Diseases, Neurological Disorders, Guillain-Barré Syndrome, Myelitis Transverse, Stroke, Patient, Peripheral Nervous System Diseases. The methodological quality was evaluated according to STROBE and the level of evidence was established according to CEBM.
Results: In the first search, 645 articles were identified. 637 were discarded by filters, titles, duplicate abstracts, methodological quality, and level of evidence. There were 8 articles selected for the present review in which neuromuscular alterations of central and peripheral origin were identified, such as myalgias, fatigue, polyneuroradiculopathies, CNS inflammation, among others, with clinical manifestations that affect movement.
Conclusion: COVID-19 is a multisystemic disease that can affect the nervous system with symptoms of neuromuscular alterations that compromise body movement.
Keywords
SARS-Cov-2, COVID-19, Long Covid, Motor activity neuromuscular diseases, neurological disorders, Guillain-Barré Syndrome, Myelitis transverse, Stroke, patient, Peripheral nervous system diseases.
Resumen
Introducción: La pandemia por la COVID-19 ha traído consecuencias a corto, mediano y largo plazo sobre la calidad de vida de los afectados. Entre las complicaciones se encuentran aquellas relacionadas con la afectación del sistema nervioso y las estructuras involucradas en el movimiento corporal, con secuelas que pueden ser transitorias y/o definitivas, y requieren rehabilitación.
Objetivo: Identificar las alteraciones neuromusculares que afectan el movimiento corporal, asociadas a la COVID-19.
Material y métodos: Se hizo una búsqueda de trabajos observacionales publicados en las bases de datos SCOPUS, PubMed, EBSCO y Nature entre enero de 2020 y junio de 2022 bajo metodología PRISMA, para dar respuesta a la pregunta PICO: ¿cuáles son las alteraciones neuromusculares que potencialmente pueden afectar el movimiento, asociadas a la COVID-19? Los filtros establecidos fueron tipo de estudio, idioma, edad, disponibilidad y fechas de publicación. Los términos MesH fueron SARS-Cov-2, COVID-19, Long Covid, Motor Activity Neuromuscular Diseases, Neurological Disorders, Guillain-Barré Syndrome, Myelitis Transverse, Stroke, Patient, Peripheral Nervous System Diseases. La calidad metodológica se evaluó según STROBE y el nivel de evidencia se estableció según CEBM.
Resultados: En la primera búsqueda se identificaron 645 artículos. Posteriormente se descartaron 637 por filtros, títulos, resúmenes duplicados, calidad metodológica y nivel de evidencia. Así, quedaron seleccionados 8 para la presente revisión, en los cuales se identificó alteraciones neuromusculares de origen central y periférico, como mialgias, fatiga, polineuroradiculopatías, inflamación del SNC, entre otras, con manifestaciones clínicas que afectan el movimiento.
Conclusión: La COVID-19 es una enfermedad multisistémica que puede afectar el sistema nervioso con síntomas de alteraciones neuromusculares que comprometen el movimiento corporal.
Palabras clave
SARS-Cov-2, COVID-19, Long Covid, Desorden de la actividad neuromuscular, desordenes neurológicos, Sindrome de Guillain Barre, Mielitis Transversa, Ataque, Paciente, Desorden del sistema nervioso periférico.
Introduction
The new coronavirus, discovered in China and identified as SARS-CoV-2, is a zoonotic- type beta coronavirus virus [1], which has an unprecedented global morbidity and mortality [2]. The Pan-American Health Organization (PAHO) [3] reported on July 1, 2022, a total of 163,260,535 cumulative cases in the Region of the Americas, of which 2,762,900 were cumulative deaths and 160,497,635 were the total of recovered cases. A study from November 24, 2021 to February 27, 2022 on the Omicron variant of SARS- CoV worldwide found an increase in cases of 41.50% [4]. One of the countries with high mortality in Latin America is Mexico, with 325,793 deaths in July 2022 [5]. In Colombia, the National Institute of Health reported for the same date 6,175,181 confirmed cases, of which 140,070 were deaths and 5,984,546 were the total of recovered cases [6].
Studies indicate that the disease caused by SARS-CoV-2, called COVID-19 by the World Health Organization (WHO), is the cause of a cytokine storm with a post-inflammatory state, which leads to vascular complications and hypercoagulability, generating an increased risk of cerebrovascular disease (CVD), involvement of structures involved in motor control with signs and symptoms of dysfunction for control, movement regulation and neuromuscular complications that affect motor skills and put the life of those who suffer from it at risk [7]. Some authors neurologically evaluated patients admitted to intensive care units (ICU) due to COVID-19 and found neuromuscular pathology (16.66%), cerebrovascular pathology (13.33%) and encephalopathies/encephalitis (13.33%) [8]. Other authors also observed that ICU stay results in greater movement restriction, due to prolonged periods in bed, which poses an additional risk of muscle loss, especially in older people [9].
Emerging alterations associated with COVID-19 with motor impairment and great lethal potential have also been identified, among which is Guillain-Barré syndrome, an autoimmune neurological disease [10], due to the formation of autoantibodies that direct their activity towards myelin sheaths [11]. On the other hand, rapid demyelination has been observed, leading to transverse myelitis [12], which usually occur in the acute period of the disease. There are other lasting symptoms in patients recovering from an acute phase and are called post-acute COVID-19 syndrome or post-acute sequelae of SARS- CoV-2 infection (PASC) or long/Covid, where several neuromuscular symptoms and manifestations are also present [13] with motor alterations.
All patients with COVID-19 are potentially at risk of developing these diseases, whose pathophysiological mechanisms are exacerbated by the autoimmune and hyperinflammatory responses of the disease, leaving sequelae susceptible to intervention from rehabilitation. Despite the impact on patients' quality of life, in the search for information for the present review, no studies were found that group or consolidate neuromuscular alterations, their sequelae or their approaches from rehabilitation. Being aware of this is essential for teams to detect complicated cases early. Therefore, it is considered that the present study can strengthen the evidence that helps save lives and reduce disability. The present review aimed to identify the neuromuscular alterations associated with COVID-19 in adult patients over 18 years of age through a review of scientific literature.
Materials and methods
A review of scientific literature was made to answer the PICO question: What are the neuromuscular alterations associated with COVID-19?
Selection criteria and search strategy
We conducted a comprehensive search of articles between June 27th 2022 and July 25th 2022 in SCOPUS, PubMed, EBSCO and Nature databases. The established limits were: publications of observational studies in full text, written in Spanish, English and Portuguese, published in the last 10 years, with emphasis on 2020-2022.
The terms MesH were used: "SARS-Cov-2", "COVID-19", "Long Covid", "Motor activity neuromuscular diseases", "Neurological disorders", "Guillain-Barré Syndrome", "Myelitis transverse", "Stroke, patient" and "Peripheral nervous system diseases". They were combined with the Bolegan operators AND or NOT and entered into the selected databases.
Inclusion criteria
We included observational studies referring to patients with COVID-19 and/or Long Covid, whose disease presented or presented with alterations of the central nervous system and/or neuromuscular, male and female, over 18 years of age, hospitalized and not hospitalized.
Exclusion criteria
Studies of patients who completed or were suffering from COVID-19 without neurological and/or neuromuscular complications were excluded; those studies that included COVID-19 patients with pre-existing neuromuscular diseases and/or alterations that could induce biases in the results.
Data extraction
The search was done separately by the researchers and the selection was made by consensus. After applying the filters and excluding titles and abstracts, duplicity, language, date of publication and not meeting inclusion criteria, the results were evaluated for methodological quality through Strengthening the Reporting of Observational studies in Epidemiology (STROBE), applying as a minimum compliance of 75% of the items (contains 22 items). The level of evidence was assessed with the protocol of the Centre for Evidence-Based Medicine of Oxford (CEBM), choosing those studies with a high level of evidence and degrees of recommendation (see Table 1). The information that allowed the answer to the PICO question was extracted.
Table 1. Characteristics of the studies included in the systematic review
| Article title | Authors | Participations (PICOS) | Intervention (PICOS) | Comparison (PICOS) | Outcome (PICOS) | Study Design (PICOS) |
|---|---|---|---|---|---|---|
| Guillain-Barré Syndrome Related and Unrelated to COVID-19: Clinical Follow-Up in the COVID-19 Era [8] | Masuccio FG, Tipa V, Invernizzi M, Solaro C. | N = 11 | Examination of patients with GBS associated with COVID-19 using the Barthel index and GBS-Disability Scale. | Patients with C- GBS and patients with NC-GBS. | 55.55% of patients with GBS associated with COVID-19 had a better clinical outcome as they recovered their gait autonomously. | Observational cohort |
| Long COVID: rheumatologic/ musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months [13] | Karaarslan F, Güneri FD, Kardeş S. | N = 300 | Description of rheumatic and musculoskeletal symptoms in COVID- 19 survivors. | Not applicable | At 3 months, 89.0% of survivors had at least one symptom, 74.6% had at least one rheumatic and musculoskeletal symptom (myalgia, arthralgia, low back pain), and 82.1% had at least one other symptom of COVID-19. At 6 months, 59.6% of survivors had at least one symptom, 43.2% had at least one rheumatic and musculoskeletal symptom (myalgia, arthralgia, low back pain), and 51.2% had at least one other symptom of COVID- 19. | Observational cohort |
| Clinical and Electrophysiological Outcome Measures of Patients With Post-Infectious Neurological Syndromes Related to COVID-19 Treated With Intensive Neurorehabilitation [14] | Avenali M, Martinelli D, Todisco M, Canavero I, Valentino F, Micieli G, et al. | N = 5 patients | Illustrating neurophysiological and functional recovery in patients with para-infection and post-infection neurological syndromes (PINS) after COVID-19 | Not applicable | Function and independence were recovered in 1 patient with acute inflammatory demyelinating polyradiculoneuropathy (AIDP) associated with COVID-19 and moderately in two of the patients presenting with AIDP and acute sensory and motor axonal neuropathy. 1 patient with myelitis showed prolonged central motor control and another had a recovery with persistence of lower limb muscle weakness. | Observational cohorte |
| Predictors of long covid 19 syndrome [15] | Mady AF, Abdelfattah RA, Kamel FMM, Abdel Naiem ASM, AbdelGhany WM, Abdelaziz AO. | N = 164 | To assess symptoms that persist after the acute stage of illness in a cohort of patients with suspected or confirmed COVID 19 and to define predictors of prolonged COVID syndrome. | Patients with prior confirmation or high suspicion of COVID-19 treated at El-Minia University Chest Hospital | The most frequently reported neuromuscular symptoms were chronic fatigue and neuropathy (15.2%); vertigo and headache (9.1%), arthralgias and myalgias (6.1%). | Retrospective cohort |
| Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19 [16] | Versace V, Sebastianelli L, Ferrazzoli D, Romanello R, Ortelli P, Saltuari L, et al. | N = 12 patients | Apply transcranial magnetic stimulation (TMS) to explore the activity of inhibitory intracortical circuits in the primary cortex (M1) in patients with fatigue and dysfunction after COVID-19 with neurological symptoms. | Patients with neurological complications after COVID-19 vs healthy patients (control) | PostCOVID-19 patients showed fatigue with the Fatigue Rating Scale with score (8.1 ± 1.7) and pathological score in the Frontal Assessment Battery (12.2 ± 0.7). Transcranial magnetic stimulation showed reduction of cortical inhibition at short intervals (p < 0.001 at 2ms and p < 0.01 at 3 ms) and disruption of cortical inhibition at long intervals (p = 0.008 at 50 ms and p < 0.001 at 100 ms) compared to healthy | Observational cohort |
| Subclinical myopathic changes in COVID-19 [17] | Villa D, Ardolino G, Borellini L, Cogiamanian F, Vergari M, Savojardo V, Peyvandi F, Barbieri S. | N = 12 patients | Assess the relationship between SARS-Cov-2 and primary neuromuscular function | Not applicable | Myopathic changes were shown with needle electromyography (6/12) prominent in the trapezius (50%), iliopsoas (33%) and deltoid (8%) muscles all asymptomatic in muscle symptoms. There were no significant differences in the laboratories in the groups. | Cohort |
| Evolving phenotypes of non- hospitalized patients that indicate long COVID [18] | Estiri H, Strasser ZH, Brat GA, Semenov YR, Aaron JR, Agapito G, et al. | N = 96.025 | Accurate identification of the phenotypes of post-acute sequelae of COVID-19 | Live and non- hospitalized patients. who underwent SARS- CoV-2 reverse transcriptase polymerase chain reaction tests | 33 phenotypes, including chronic fatigue and muscle weakness, were identified as more frequent post-acute sequelae of COVID-19 (PASC) in non-hospitalized patients. | Retrospective Cohort |
| Myopathic changes in patients with long-term fatigue after COVID-19 [19] | Agergaard J, Leth S, Pedersen TH, Harbo T, Blicher JU, Karlsson P, et al. | N = 20 patients | Investigating peripheral nerve and electrophysiological muscle function in patients with persistent neuromuscular symptoms after COVID-19 | Long Covid patients with persistent neuromuscular symptoms vs control of healthy patients of the same sex and age | Motor unit potential was reduced in the biceps brachii, vastus medial and tibialis anterior compared to control (p< 0.0001); Physical fatigue and myalgia were more common in patients with electromyography compared to the control group (p< 0.05) | Observational cohort |
Note. N = sample number.
Results
Results Search
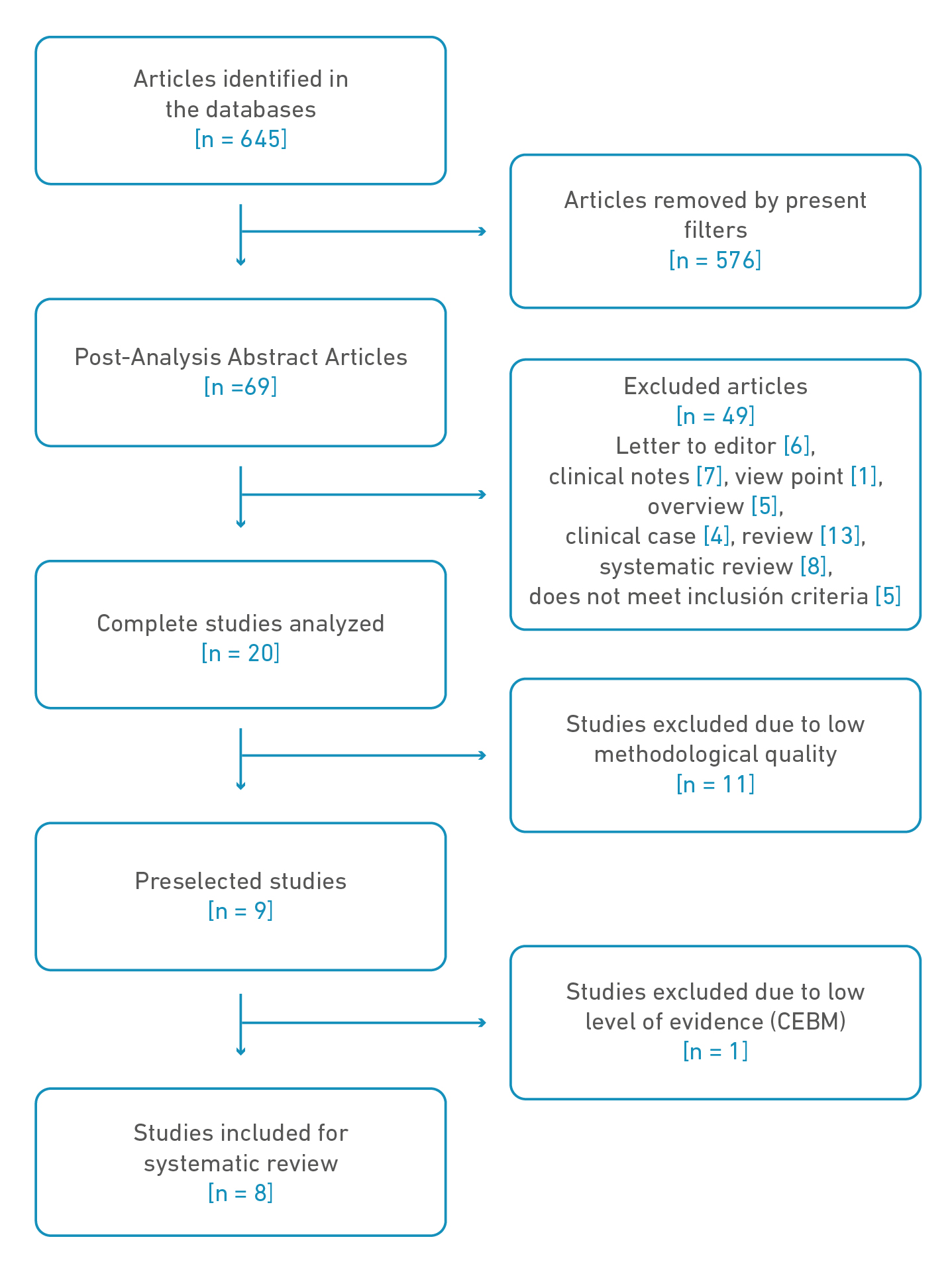
At the end of the selection of the first search, a total of 645 articles were obtained (PubMed, 74; SCOPUS, 185; EBSCO, 76; Nature, 310). After going through the different filters, 69 articles remained for analysis (PubMed, 23; SCOPUS, 20; EBSCO, 22; Nature, 4). De these were excluded 49 for not meeting criteria, leaving 20, to which evaluation of the methodological quality was made, leaving 9 preselected. We excluded 1 and selected 8 for the present review (Figure 1).
Figure 1. Diagram, search, sorting, and item selection
Study characteristics
Risk of bias
In terms of risk of bias, we found that methods differed for each study, which could lead to bias. However, each author made the mechanisms explicit.
Heterogeneity analysis
We found that the studies analyzed differed in terms of population selection and measurement instruments.
Synthesis of results
The present review allowed us to identify that, associated with COVID-19, there are transient and/or definitive neuromuscular alterations of central and/or peripheral origin that affect movement, and therefore the functionality and functioning of those who suffer from it [8,13-19]. About that:
The objective of Masuccio et al. [8] was to establish the outcome of patients with GBS associated with COVID-19, using the Barthel index and disability of 11 patients. In this cohort, a higher than expected prevalence of acute motor axonal neuropathy was detected. The COVID-19-related GBS group did not have any complications. After a mean of 10.11 months (DE = 4.46 months), 55.55% of patients regained autonomous gait.
The aim of Karaarslan et al. [13] was to document characteristics related to the severity, type, and location of rheumatic and musculoskeletal symptoms in 300 COVID-19 survivors aged 18 to 70 years. They found at three months that 74.6% had at least one rheumatic and musculoskeletal symptom (myalgia, arthralgia, low back pain); and at 6 months, 43.2% had at least one rheumatic and musculoskeletal symptom (myalgia, arthralgia, low back pain). These manifestations are mainly due to changes at the musculoskeletal level manifested by muscle atrophy, and the limitations for movements were partly produced by physical inactivity as a consequence of the disease [8].
The objective of Avenali et al. [14] was to illustrate neurophysiological and functional recovery in 5 patients with para-infection and post-infection neurological syndromes (PINS) after COVID-19, who were followed for six months. The group of patients presented with inflammation of the central and peripheral nervous system in response to SarsCov2 infection, manifested by polyneuroradiculopathy, acute motor and sensory axonal neuropathy, transverse myelitis. They concluded that patients with peripheral involvement had a worse prognosis than those at the central level.
The objective of Mady et al. [15] was to assess symptoms that persisted after the acute stage of disease in 164 patients with more than three months of disease progression. They identified chronic fatigue and neuropathy (15.2%), chronic dyspnea in 9.8%, mostly in severe cases, vertigo and headache in 9.1%, musculoskeletal symptoms in 6.1% and skin lesions in 3.7%.
The objective of Versace et al. [16] was to explore the activity of major inhibitory intracortical circuits within the primary motor cortex (M1) in a sample of 12 patients complaining of fatigue and presenting with executive dysfunction after resolution of COVID-19 with neurological manifestations. They observed changes in cortical synaptic currents that may develop persistent symptoms even after the disease, such as reduction in inhibition of the primary motor cortex (M1), as disruption of GABA energetic intracortical circuits (responsible for supplying signals for functional output of the brain), mediated by short-interval intracortical inhibition through transcranial magnetic stimulation (p < 0.001), with motor symptoms such as fatigue and some alterations in executive functions.
The objective of Villa et al. [17] was to assess the extent of primary neuromuscular involvement in SarsCov2 infection in a group of 70 patients aged 18 to 70 years, 12 of whom did not have severe disease at three-week follow-up. They found myopathic changes with needle electromyography (6/12) prominent in the trapezius (50%), iliopsoas (33%) and deltoid (8%) muscles; all asymptomatic in muscular symptoms.
The objective of Estiri et al. [18] was to identify the phenotypes of post-acute sequelae of COVID-19 in 96,025 subjects. We identified 33 phenotypes among different age/gender cohorts or time windows that were positively associated with a previous SARS-CoV infection. These phenotypes include chest pain (OR 1.27, 95% CI [1.09- 1.48]), chronic fatigue syndrome (OR 2.60, 95% CI [1.22-2.10]), shortness of breath (OR 1.41, 95% CI [1.22-1.64]), pneumonia (OR 1.66, 95% CI [1.28-2.16])). In addition, type 2 diabetes mellitus (OR 1.41, 95% CI [1.22-1.64]) is one of the most significant indicators of a past COVID-19 infection. On the other hand, more new phenotypes were found with greater confidence among cohorts younger than 65 years.
The objective of Agergaard et al. [19] was to investigate peripheral nerve and electrophysiological muscle function in 20 patients with persistent neuromuscular symptoms after COVID-19. Results included reduction in motor unit potential in the biceps brachii, vastus medial and tibialis anterior, compared to control (p < 0.0001); physical fatigue and myalgia, compared to the control group, was more common in patients with electromyography (p < 0.05).
Neurological problems affecting motor activity had in some studies a worse diagnosis and prognosis in patients hospitalized in ICU. Frontera et al. [20] reported that 91% of patients had at least one abnormal result in six months and those with neurological complications during hospitalization were unable to comply with activities of daily living (CI 0.29-.74, p=0.01) and only 41% were able to return to work normally during that period, compared to 64% of patients without neurological complications.
Discussion
In the present review, neuromuscular alterations were identified in patients with COVID- 19 as a complication derived from it, although with different mechanisms of action; involvement with signs and symptoms of different structures of the central and peripheral nervous system, which affects the control and regulation of movement. Among the manifestations were diseases that have their own physio-pathogenesis exacerbated by the infectious and hyperinflammatory state.
Neuromuscular alterations may be due to the penetration of SARS-CoV-2 into neurons, which can alter their cellular processes, such as mitochondrial dysfunction and lysosome damage, by inducing an increase in reactive oxygen species (ROS), protein misfolding/aggregation, and ultimately cell death [11,21]. This makes the SARS-CoV-2 virus not strictly neurotropic, unlike other coronaviruses previously found [22,23].
However, COVID-19 infection has been associated with a variety of neuromuscular complications ranging from nonspecific and moderate symptoms, such as headache, myalgia, and hyposmia, to severe symptoms, such as cerebrovascular disease, encephalitis, encephalopathy, multiple sclerosis, mobility and ataxia disorders, peripheral neuropathy, neuromuscular junction disease, and muscle disorders [23,24 ], most identified in the present study, which may occur during the water phase of infection or as post-viral manifestations [6].
CVD was identified in one of the affectations of central origin. Some authors such as Wirth et al. [25] observed that these occurred in younger subjects with moderate to severe disease and in 1-5% of hospitalized COVID-19 patients, because the novel coronavirus induces a hypercoagulable state, causing elevated D-dimer and fibrinogen levels. Another possible mechanism is represented by endothelial dysfunction, as a consequence of the depletion of the angiotensin-converting enzyme by directly infecting endothelial cells; angiotensin II could not be counteracted, producing a pro-inflammatory state, vasoconstriction, and promoting tissue injury also in the brain and its vessels [25,26]. These patients may present with alterations in motor control, such as hemiplegia and ataxia, including a reduced level of consciousness [27].
Encephalopathies have been reported in one-third of hospitalized patients, especially adults. It may be due to significant lung disease or multiorgan involvement, hypoxic or metabolic abnormalities [22].
Dyspnea with mechanical respiratory alteration is due to overstimulation of skeletal muscle. This modified metabolic situation causes excessive respiratory impulse with restriction of lung functions [28]. They may develop nonspecific symptoms, such as headache, altered consciousness, visual disturbances, seizures, memory loss, slow processing speed, delirium, or even coma [27,29]. Recently this condition has had an important recovery, thanks to thoracic physiotherapy and neurorehabilitation [30,31].
In transverse myelitis (TM) due to SARS-CoV-2 virus infection, the patient presents with paresthesias, acute flaccid weakness, and low back pain [32]. It can rapidly progress to paraplegia with total anesthesia below a certain level of the spinal cord [28]. Physiologically, this virus has neuroinvasive and neurotropic properties, causing neuronal dissemination, attack, and demyelination in the CNS to precipitate [12]. The severity of the disease is related to advanced age, respiratory and cardiovascular diseases, hypertension, diabetes and immunodeficiency patients [20].
Guillain-Barré syndrome (GBS) was also found as a pathology associated with COVID- 19, which is the result of molecular cell mimicry and formation of antibodies that target proteoglycans found in myelin sheaths [11], which is characterized by progressive ascending symmetrical weakness, areflexia and sensory loss [10]. Post-infectious GBS associated with COVID-19 is due to an exaggerated immune response, with inflammatory markers and elevated pro-inflammatory cytokines. In this type of GBS the same clinical manifestations occur [7]. Altered motor signs, axonal damage, and cytologic albumin dissociation in cerebrospinal fluid should be noted to diagnose GBS [32-33].
Early intervention in neurological complications such as GBS and myelitis may result in a better prognosis. Even so, there may be considerable axonal damage that hinders rapid recovery, coupled with immobility in some patients that may alter the results in quantitative motor potential [16,21].
GBS and TM can coexist in a Long-Covid patient. This is called GBS/TM overlap syndrome, which may include elements of GBS, such as flaccid paralysis, motor weakness, hyporeflexia or areflexia, and in TM: pyramidal signs and sensory dysfunction [32].
Chronic fatigue syndrome complicates markedly complication those patients who have developed Long-Covid, especially the female sex [14,7], being the most relevant exponent due to the absence of recovery [33]. Thus, it is due to the presence of post- inflammatory cytokines, such as interferon (IFN-γ), which when crossing the blood-brain barrier, direct their action on the hypothalamus, generating deep and disabling fatigue, long-term myalgia, reduction of daily activity and post-exertion discomfort [13,21]. Some studies indicate a prevalence rate of chronic fatigue syndrome of 16.8%. The most frequently identified symptoms are general post-exertional fatigue, headache, insomnia, dysosmia, dysgeusia, dizziness, among others [34].
Other findings directly involve skeletal muscle, often associated with decreased physical activity (PA), which directly affects musculoskeletal physiology. In this regard, some findings suggest that cytokine storm, along with the expression of coagulopathies and impaired macrophage activity, may be the reason for muscle cell damage [19,35]. They have also suggested that myopathies and sensory disturbances in some patients may be due to mitochondrial stress and microthrombi that may be expressed up to 40 days after COVID-19 [36].
There are multiple respiratory complications and systems, including those of the neuromuscular system, that a patient with SarsCov2 can suffer, which undoubtedly represents a deterioration of their quality of life. Rehabilitation will always be an opportunity for the patient's recovery [37] and his return to daily life.
Conclusions
There is evidence to understand the role of COVID-19 in the development of neuromuscular alterations; the presence or not of these may be due to certain risk factors: severity of infection, the time the patient was in ICU and the timely identification of symptoms. However, studies are limited by a field that only covers respiratory symptoms, leaving unfinished the pathophysiological mechanisms of the musculoskeletal system and the central and peripheral nervous system. Researchers need to focus their attention on investing knowledge to respond to needs for identification, early diagnosis, prognosis, treatment, along with appropriate and timely rehabilitation. All to minimize the consequences on the quality of life of affected patients. It is recommended to do prospective work to obtain information related to the topic.
References
1. Delafontaine A, Ditcharles S, Hussein T, Hoffschir M, Plantefève G, Michon D. Physiotherapy and COVID-19: A major public health role to short, medium and long terms in the patient’s rehabilitation process. Kinesitherapie. 2020;20(223):11-18. doi: https://doi.org/10.1016/j.kine.2020.05.005
2. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med [Internet]. 2021;27(4):601-15. doi: https://doi.org/10.1038/s41591-021-01283-z
3. Organización Panamericana de la Salud (OPS). Enfermedad por el Coronavirus (COVID-19) [Internet]. Paho.org. [citado el 5 de julio de 2022]. Disponible en:
4. Mora D. Impacto de la Variante Ómicron Del Sars-Cov-2 en el mundo. RADA [Internet]. 26 de julio de 2022 [citado 9 de diciembre de 2022];5(1):353-70. Disponible en: https://revistas.utn.ac.cr/index.php/arje/article/view/527
5. Agarwal A, Pinho M, Raj K, Yu FF, Bathla G, Achilleos M, et al. Neurological emergencies associated with COVID-19: stroke and beyond. Emerg Radiol [Internet]. 2020;27(6):747-54. doi: https://doi.org/10.1007/s10140-020-01837-7
6. Abenza-Abildúa MJ, Ramírez-Prieto MT, Moreno-Zabaleta R, Arenas-Valls N, Salvador-Maya MA, Algarra-Lucas C, et al. Neurological complications in critical patients with COVID-19. Neurología (English Edition) [Internet]. 2020 Nov;35(9):621-7. doi: http://dx.doi.org/10.1016/j.nrleng.2020.07.012
7. Sagarra-Romero L, Viñas-Barros A. COVID-19: Short and Long-Term Effects of Hospitalization on Muscular Weakness in the Elderly. International Journal of Environmental Research and Public Health [Internet]. 2020 Nov 24;17(23):8715. doi: http://dx.doi.org/10.3390/ijerph17238715
8. Masuccio FG, Tipa V, Invernizzi M, Solaro C. Guillain-Barré Syndrome Related and Unrelated to COVID-19: Clinical Follow-Up in the COVID-19 Era. Phys Ther. 2022 Jun 3;102(6):pzac049. doi: https://doi.org/10.1093/ptj/pzac049
9. Kirwan, R., McCullough, D., Butler, T. et al. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. GeroScience. 2020;42:1547-1578. doi: https://doi.org/10.1007/s11357-020-00272-3
10. Fumery T, Baudar C, Ossemann M, London F. Longitudinally extensive transverse myelitis following acute COVID-19 infection. Mult Scler Relat Disord. 2021 Feb;48:102723. doi: https://doi.org/10.1016/j.msard.2020.102723
11. Shimohata T. Neuro‐COVID‐19. Clinical and Experimental Neuroimmunology [Internet]. 2021 Sep 29;13(1):17-23. doi: http://dx.doi.org/10.1111/cen3.12676
12. Wong T., Weitzer D. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina MDPI. 2021;54:418. doi: https://doi.org/10.3390/medicina57050418
13. Karaarslan F, Güneri FD, Kardeş S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clinical Rheumatology [Internet]. 2021 Oct 29;41(1):289-96. doi: http://dx.doi.org/10.1007/s10067-021-05942-x
14. Avenali M, Martinelli D, Todisco M, Canavero I, Valentino F, Micieli G, et al. Clinical and Electrophysiological Outcome Measures of Patients With Post-Infectious Neurological Syndromes Related to COVID-19 Treated With Intensive Neurorehabilitation. Front Neurol. 2021;12. doi: https://doi.org/10.3389/fneur.2021.643713
15. Mady AF, Abdelfattah RA, Kamel FMM, Abdel Naiem ASM, AbdelGhany WM, Abdelaziz AO. Predictors of long covid 19 syndrome. Egypt J Hosp Med [Internet]. 2021;85(2):3604-8. doi: https://doi.org/10.21608/ejhm.2021.201970
16. Versace V, Sebastianelli L, Ferrazzoli D, Romanello R, Ortelli P, Saltuari L, et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin Neurophysiol. 2021;132(5):1138-1143. doi: https://doi.org/10.1016/j.clinph.2021.03.001
17. Villa D, Ardolino G, Borellini L, Cogiamanian F, Vergari M, Savojardo V, Peyvandi F, Barbieri S. Subclinical myopathic changes in COVID-19. Neurol Sci. 2021;42(10):3973-3979. doi: https://doi.org/10.1007/s10072-021-05469-8
18. Estiri H, Strasser ZH, Brat GA, Semenov YR, Aaron JR, Agapito G, et al. Evolving phenotypes of non-hospitalized patients that indicate long COVID. BMC Medicine [Internet]. 2021 Sep 27;19(1). doi: http://dx.doi.org/10.1186/s12916-021-02115-0
19. Agergaard J, Leth S, Pedersen TH, Harbo T, Blicher JU, Karlsson P, et al. Myopathic changes in patients with long-term fatigue after COVID-19. Clinical Neurophysiology [Internet]. 2021 Aug;132(8):1974-81. doi: http://dx.doi.org/10.1016/j.clinph.2021.04.009
20. Frontera JA, Yang D, Lewis A, Patel P, Medicherla C, Arena V, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. Journal of the Neurological Sciences [Internet]. 2021 Jul;426:117486. doi: http://dx.doi.org/10.1016/j.jns.2021.117486
21. Abboud H, Abboud FZ, Kharbouch H, Arkha Y, El Abbadi N, El Ouahabi A. COVID-19 and SARS-Cov-2 Infection: Pathophysiology and Clinical Effects on the Nervous System. World Neurosurgery [Internet]. 2020 Aug;140:49-53. doi: http://dx.doi.org/10.1016/j.wneu.2020.05.193
22. Vidale S. Risk Factors, and Clinical and Etiological Characteristics of Ischemic Strokes in COVID-19-Infected Patients: A Systematic Review of Literature. Cerebrovascular Diseases [Internet]. 2021;50(4):371-4. doi: http://dx.doi.org/10.1159/000514267
23. Haffke M, Freitag H, Rudolf G, Seifert M, Doehner W, Scherbakov N, et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). Journal of Translational Medicine [Internet]. 2022 Mar 22;20(1). doi: http://dx.doi.org/10.1186/s12967-022-03346-2
24. Fotuhi M, Mian A, Meysami S, Raji CA. Neurobiology of COVID-19. Journal of Alzheimer’s Disease [Internet]. 2020 Jun 30;76(1):3-19. Disponible en: http://dx.doi.org/10.3233/jad-200581
25. Wirth KJ, Scheibenbogen C. Dyspnea in Post-COVID Syndrome following Mild Acute COVID-19 Infections: Potential Causes and Consequences for a Therapeutic Approach. Medicina [Internet]. 2022 Mar 12;58(3):419. doi: http://dx.doi.org/10.3390/medicina58030419
26. Harapan BN, Yoo HJ. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19). Journal of Neurology [Internet]. 2021 Jan 23;268:3059-3071. doi: http://dx.doi.org/10.1007/s00415-021-10406-y
27. Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: An overview. Diabetes & Metabolic Syndrome: Clinical Research & Reviews [Internet]. 2021 May;15(3):869-75. doi: http://dx.doi.org/10.1016/j.dsx.2021.04.007
28. Ahmad SJ, Feigen CM, Vazquez JP, Kobets AJ, Altschul DJ. Neurological Sequelae of COVID-19. Journal of Integrative Neuroscience [Internet]. 2022 Apr 6;21(3):77. doi: http://dx.doi.org/10.31083/j.jin2103077
29. Alrubaye R, Bondugula V, Baleguli V, Chofor R. A possible Guillain-Barré syndrome/transverse myelitis overlap syndrome after recent COVID-19. BMJ Case Reports [Internet]. 2022 Feb;15(2):e246967. doi: http://dx.doi.org/10.1136/bcr-2021-246967
30. Raahimi MM, Kane A, Moore CE, Alareed AW. Late onset of Guillain-Barré syndrome following SARS-CoV-2 infection: part of ‘long COVID-19 syndrome’? BMJ Case Reports [Internet]. 2021 Jan;14(1):e240178. doi: http://dx.doi.org/10.1136/bcr-2020-240178
31. Vallejo Serna R, Cantor González JF, Arce Gálvez L. Síndrome de Guillain-Barré asociado a COVID-19: diagnóstico, tratamiento y rehabilitación. Neurology Perspectives [Internet]. 2021 Jan;1(1):104-6. doi: http://dx.doi.org/10.1016/j.neurop.2021.03.003
32. Sundar Shrestha D, Love R. Long COVID Patient Symptoms and its Evaluation and Management. Journal of Nepal Medical Association [Internet]. 2021 Aug 12;59(240). doi: http://dx.doi.org/10.31729/jnma.6355
33. Bax F, Lettieri C, Marini A, Pellitteri G, Surcinelli A, Valente M, et al. Clinical and neurophysiological characterization of muscular weakness in severe COVID-19. Neurological Sciences [Internet]. 2021 Mar 23;42(6):2173-8. doi: http://dx.doi.org/10.1007/s10072-021-05110-8
34. Tokumasu K, Honda H, Sunada N, Sakurada Y, Matsuda Y, Yamamoto K, et al. Clinical Characteristics of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Diagnosed in Patients with Long COVID. Medicina [Internet]. 2022 Jun 25;58(7):850. Available from: http://dx.doi.org/10.3390/medicina58070850
35. Chakravarty N, Senthilnathan T, Paiola S, Gyani P, Castillo Cario S, Urena E, et al. Neurological pathophysiology of SARS‐CoV‐2 and pandemic potential RNA viruses: a comparative analysis. FEBS Letters [Internet]. 2021 Nov 22;595(23):2854-71. doi: http://dx.doi.org/10.1002/1873-3468.14227
36. Nath A, Smith B. Neurological issues during COVID-19: An overview. Neuroscience Letters [Internet]. 2021 Jan;742:135533. doi: http://dx.doi.org/10.1016/j.neulet.2020.135533
37. Almanza-Díaz Y, Carmona-Ferrer B, Sabater-Hernández H. Consideraciones sobre rehabilitación pos-COVID-19. Revista Cubana de Medicina Física y Rehabilitación [revista en Internet]. 2022 [citado 24 Oct 2022];14(3). Disponible en: http://www.revrehabilitacion.sld.cu/index.php/reh/article/view/784